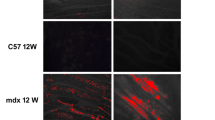
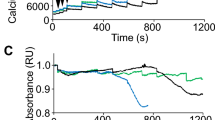
Abstract
Abnormal intracellular Ca2+ handling is an important factor in the progressive functional decline of dystrophic muscle. In the present study, we investigated the function of sarco(endo)plasmic reticulum (SR) Ca2+ ATPase (SERCA) in various dystrophic muscles of mouse models of Duchenne muscular dystrophy. Our studies show that the protein expression of sarcolipin, a key regulator of the SERCA pump is abnormally high and correlates with decreased maximum velocity of SR Ca2+ uptake in the soleus, diaphragm and quadriceps of mild (mdx) and severe (mdx:utr−/−) dystrophic mice. These changes are more pronounced in the muscles of mdx:utr−/− mice. We also found increased expression of SERCA2a and calsequestrin specifically in the dystrophic quadriceps. Immunostaining analysis further showed that SERCA2a expression is associated both with fibers expressing slow-type myosin and regenerating fibers expressing embryonic myosin. Together, our data suggest that sarcolipin upregulation is a common secondary alteration in all dystrophic muscles and contributes to the abnormal elevation of intracellular Ca2+ concentration via SERCA inhibition.




Similar content being viewed by others
References
Alderton JM, Steinhardt RA (2000) Calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem 275(13):9452–9460
Asahi M, Otsu K, Nakayama H, Hikoso S, Takeda T, Gramolini AO, Trivieri MG, Oudit GY, Morita T, Kusakari Y, Hirano S, Hongo K, Hirotani S, Yamaguchi O, Peterson A, Backx PH, Kurihara S, Hori M, MacLennan DH (2004) Cardiac-specific overexpression of sarcolipin inhibits sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2a) activity and impairs cardiac function in mice. Proc Natl Acad Sci USA 101(25):9199–9204. doi:10.1073/pnas.0402596101
Babu GJ, Bhupathy P, Petrashevskaya NN, Wang H, Raman S, Wheeler D, Jagatheesan G, Wieczorek D, Schwartz A, Janssen PM, Ziolo MT, Periasamy M (2006) Targeted overexpression of sarcolipin in the mouse heart decreases sarcoplasmic reticulum calcium transport and cardiac contractility. J Biol Chem 281(7):3972–3979. doi:10.1074/jbc.M508998200
Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M (2007a) Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol 43(2):215–222. doi:10.1016/j.yjmcc.2007.05.009
Babu GJ, Bhupathy P, Timofeyev V, Petrashevskaya NN, Reiser PJ, Chiamvimonvat N, Periasamy M (2007b) Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci USA 104(45):17867–17872. doi:10.1073/pnas.0707722104
Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR (2009) Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15(3):325–330. doi:10.1038/nm.1916
Bhupathy P, Babu GJ, Periasamy M (2007) Sarcolipin and phospholamban as regulators of cardiac sarcoplasmic reticulum Ca2+ ATPase. J Mol Cell Cardiol 42(5):903–911. doi:10.1016/j.yjmcc.2007.03.738
Bonilla E, Samitt CE, Miranda AF, Hays AP, Salviati G, DiMauro S, Kunkel LM, Hoffman EP, Rowland LP (1988) Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell 54(4):447–452
Capote J, DiFranco M, Vergara JL (2010) Excitation-contraction coupling alterations in mdx and utrophin/dystrophin double knockout mice: a comparative study. Am J Physiol Cell Physiol 298(5):C1077–1086. doi:10.1152/ajpcell.00428.2009
Collet C, Allard B, Tourneur Y, Jacquemond V (1999) Intracellular calcium signals measured with indo-1 in isolated skeletal muscle fibres from control and mdx mice. J Physiol 520(Pt 2):417–429
Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE (1997) Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 90(4):717–727. doi:10.1073/pnas.0802217105
Divet A, Huchet-Cadiou C (2002) Sarcoplasmic reticulum function in slow- and fast-twitch skeletal muscles from mdx mice. Pflugers Arch 444(5):634–643. doi:10.1007/s00424-002-0854-5
Divet A, Lompre AM, Huchet-Cadiou C (2005) Effect of cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca-ATPase, on skeletal muscles from normal and mdx mice. Acta Physiol Scand 184(3):173–186. doi:10.1111/j.1365-201X.2005.01450.x
Edwards JN, Friedrich O, Cully TR, von Wegner F, Murphy RM, Launikonis BS (2010) Upregulation of store-operated Ca2+ entry in dystrophic mdx mouse muscle. Am J Physiol Cell Physiol 299(1):C42–50. doi:10.1152/ajpcell.00524.2009
Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, Marks AR, Lacampagne A (2010) Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci USA 107(4):1559–1564. doi:10.1073/pnas.0908540107
Ferretti R, Marques MJ, Pertille A, Santo Neto H (2009) Sarcoplasmic-endoplasmic-reticulum Ca2+-ATPase and calsequestrin are overexpressed in spared intrinsic laryngeal muscles of dystrophin-deficient mdx mice. Muscle Nerve 39(5):609–615. doi:10.1002/mus.21154
Gehrig SM, Koopman R, Naim T, Tjoakarfa C, Lynch GS (2010) Making fast-twitch dystrophic muscles bigger protects them from contraction injury and attenuates the dystrophic pathology. Am J Pathol 176(1):29–33. doi:10.2353/ajpath.2010.090760
Gehrig SM, van der Poel C, Sayer TA, Schertzer JD, Henstridge DC, Church JE, Lamon S, Russell AP, Davies KE, Febbraio MA, Lynch GS (2012) Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature 484(7394):394–398. doi:10.1038/nature10980
Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, Kranias EG, Molkentin JD (2011) Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest 121(3):1044–1052. doi:10.1172/JCI43844
Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR (1997) Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 90(4):729–738
Hoffman EP, Brown RH Jr, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51(6):919–928
Kargacin ME, Kargacin GJ (1996) The sarcoplasmic reticulum calcium pump is functionally altered in dystrophic muscle. Biochim Biophys Acta 1290(1):4–8
Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA (2001) Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol 535(Pt 2):591–600
MacLennan DH, Asahi M, Tupling AR (2003) The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann N Y Acad Sci 986:472–480
Marotta M, Ruiz-Roig C, Sarria Y, Peiro JL, Nunez F, Ceron J, Munell F, Roig-Quilis M (2009) Muscle genome-wide expression profiling during disease evolution in mdx mice. Physiol Genomics 37(2):119–132. doi:10.1152/physiolgenomics.90370.2008
Matsumura K, Ervasti JM, Ohlendieck K, Kahl SD, Campbell KP (1992) Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 360(6404):588–591. doi:10.1038/360588a0
Moens P, Baatsen PH, Marechal G (1993) Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J Muscle Res Cell Motil 14(4):446–451
Periasamy M, Kalyanasundaram A (2007) SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve 35(4):430–442. doi:10.1002/mus.20745
Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90(8):3710–3714
Robin G, Berthier C, Allard B (2012) Sarcoplasmic reticulum Ca2+ permeation explored from the lumen side in mdx muscle fibers under voltage control. J Gen Physiol 139(3):209–218. doi:10.1085/jgp.201110738
Rouger K, Le Cunff M, Steenman M, Potier MC, Gibelin N, Dechesne CA, Leger JJ (2002) Global/temporal gene expression in diaphragm and hindlimb muscles of dystrophin-deficient (mdx) mice. Am J Physiol Cell Physiol 283(3):C773–784. doi:10.1152/ajpcell.00112.2002
Shin DW, Pan Z, Kim EK, Lee JM, Bhat MB, Parness J, Kim DH, Ma J (2003) A retrograde signal from calsequestrin for the regulation of store-operated Ca2+ entry in skeletal muscle. J Biol Chem 278(5):3286–3292. doi:10.1074/jbc.M209045200
Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ (1989) The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244(4912):1578–1580
Tkatchenko AV, Le Cam G, Leger JJ, Dechesne CA (2000) Large-scale analysis of differential gene expression in the hindlimb muscles and diaphragm of mdx mouse. Biochim Biophys Acta 1500(1):17–30
Tupling AR (2009) The decay phase of Ca2+ transients in skeletal muscle: regulation and physiology. Appl Physiol Nutr Metab 34(3):373–376. doi:10.1139/H09-033
Tupling AR, Asahi M, MacLennan DH (2002) Sarcolipin overexpression in rat slow twitch muscle inhibits sarcoplasmic reticulum Ca2+ uptake and impairs contractile function. J Biol Chem 277(47):44740–44746. doi:10.1074/jbc.M206171200
Tupling AR, Bombardier E, Gupta SC, Hussain D, Vigna C, Bloemberg D, Quadrilatero J, Trivieri MG, Babu GJ, Backx PH, Periasamy M, MacLennan DH, Gramolini AO (2011) Enhanced Ca2+ transport and muscle relaxation in skeletal muscle from sarcolipin-null mice. Am J Physiol Cell Physiol 301(4):C841–849. doi:10.1152/ajpcell.00409.2010
Turner PR, Westwood T, Regen CM, Steinhardt RA (1988) Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335(6192):735–738. doi:10.1038/335735a0
Turner PR, Fong PY, Denetclaw WF, Steinhardt RA (1991) Increased calcium influx in dystrophic muscle. J Cell Biol 115(6):1701–1712
Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P (2002) Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol 158(6):1089–1096. doi:10.1083/jcb.200203091
Verma S, Anziska Y, Cracco J (2010) Review of Duchenne muscular dystrophy (DMD) for the pediatricians in the community. Clin Pediatr (Phila) 49(11):1011–1017. doi:10.1177/0009922810378738
Webster C, Silberstein L, Hays AP, Blau HM (1988) Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 52(4):503–513
Woods CE, Novo D, DiFranco M, Vergara JL (2004) The action potential-evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibres. J Physiol 557(Pt 1):59–75. doi:10.1113/jphysiol.2004.061291
Acknowledgments
This work was supported by the funds from Department of Cell Biology and Molecular Medicine, UMDNJ-NJMS, Newark, NJ (to G.J.B. and D.F.). D.F. is supported by the Muscular Dystrophy Association (200037) and by the Hispanic Center of Excellence. J.S.S. is supported by a NIH training Grant (T32HL069752).
Author information
Authors and Affiliations
Corresponding author
Additional information
Joel S. Schneider and Mayilvahanan Shanmugam contributed equally to this study.
Rights and permissions
About this article
Cite this article
Schneider, J.S., Shanmugam, M., Gonzalez, J.P. et al. Increased sarcolipin expression and decreased sarco(endo)plasmic reticulum Ca2+ uptake in skeletal muscles of mouse models of Duchenne muscular dystrophy. J Muscle Res Cell Motil 34, 349–356 (2013). https://doi.org/10.1007/s10974-013-9350-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-013-9350-0