Abstract
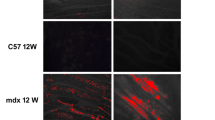
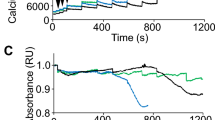
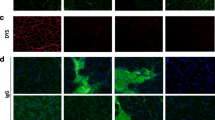
Skeletal muscles of patients with Duchenne muscular dystrophy (DMD) show numerous alterations including inflammation, apoptosis, and necrosis of myofibers. However, the molecular mechanism that explains these changes remains largely unknown. Here, the involvement of hemichannels formed by connexins (Cx HCs) was evaluated in skeletal muscle of mdx mouse model of DMD. Fast myofibers of mdx mice were found to express three connexins (39, 43 and 45) and high sarcolemma permeability, which was absent in myofibers of mdx Cx43fl/flCx45fl/fl:Myo-Cre mice (deficient in skeletal muscle Cx43/Cx45 expression). These myofibers did not show elevated basal intracellular free Ca2+ levels, immunoreactivity to phosphorylated p65 (active NF-κB), eNOS and annexin V/active Caspase 3 (marker of apoptosis) but presented dystrophin immunoreactivity. Moreover, muscles of mdx Cx43fl/flCx45fl/fl:Myo-Cre mice exhibited partial decrease of necrotic features (big cells and high creatine kinase levels). Accordingly, these muscles showed similar macrophage infiltration as control mdx muscles. Nonetheless, the hanging test performance of mdx Cx43fl/flCx45fl/fl:Myo-Cre mice was significantly better than that of control mdx Cx43fl/flCx45fl/fl mice. All three Cxs found in skeletal muscles of mdx mice were also detected in fast myofibers of biopsy specimens from patients with muscular dystrophy. Thus, reduction of Cx expression and/or function of Cx HCs may be potential therapeutic approaches to abrogate myofiber apoptosis in DMD.











Similar content being viewed by others
References
Emery AE (2002) Muscular dystrophy into the new millennium. Neuromuscul Disord 12:343–349
Ervasti JM, Campbell KP (1993) A role for the dystrophin–glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol 122:809–823
Pillers D (2014) A new day for Duchenne’s? The time has come for newborn screening. Mol Genet Metab 113:11–13
Bulfield G, Siller WG, Wight PA, Moore KJ (1984) X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81:1189–1192
Balnave CD, Allen DG (1995) Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J Physiol 488:25–36
Yeung EW, Head SI, Allen DG (2003) Gadolinium reduces short-term stretch-induced muscle damage in isolated mdx mouse muscle fibres. J Physiol 552:449–458
Matsuda R, Nishikawa A, Tanaka H (1995) Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J Biochem 118:959–964
Hamer PW, McGeachie JM, Davies MJ, Grounds MD (2002) Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat 200:69–79
Cea LA, Cisterna BA, Puebla C, Frank M, Figueroa XF, Cardozo C et al (2013) De novo expression of connexin hemichannels in denervated fast skeletal muscles leads to atrophy. Proc Natl Acad Sci USA 110:16229–16234
Sáez JC, Leybaert L (2014) Hunting for connexin hemichannels. FEBS Lett 588:1205–1211
Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD (2002) Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol 185:93–102
Franco A Jr, Lansman JB (1990) Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature 344:670–673
Bao L, Sachs F, Dahl G (2004) Connexins are mechanosensitive. Am J Physiol Cell Physiol 287:C1389–C1395
Fiori MC, Figueroa V, Zoghbi ME, Saéz JC, Reuss L, Altenberg GA (2012) Permeation of calcium through purified connexin 26 hemichannels. J Biol Chem 287:40826–40834
Schalper KA, Sánchez HA, Lee SC, Altenberg GA, Nathanson MH, Sáez JC (2010) Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. Am J Physiol Cell Physiol 299:C1504–C1515
Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S (2009) Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Genet 18:824–834
Valladares D, Almarza G, Contreras A, Pavez M, Buvinic S, Jaimovich E et al (2013) Electrical stimuli are anti-apoptotic in skeletal muscle via extracellular ATP. Alteration of this signal in mdx mice is a likely cause of dystrophy. PLoS One 8:e75340
Yeung D, Zablocki K, Lien CF, Jiang T, Arkle S, Brutkowski W et al (2006) Increased susceptibility to ATP via alteration of P2X receptor function in dystrophic mdx mouse muscle cells. FASEB J 20:610–620
Young CN, Brutkowski W, Lien CF, Arkle S, Lochmüller H, Zabłocki K et al (2012) P2X7 purinoceptor alterations in dystrophic mdx mouse muscles: relationship to pathology and potential target for treatment. J Cell Mol Med 16:1026–1037
Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI (2000) Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol 32:1859–1872
Sáez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV (2010) Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res 316:2377–2389
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Perálvarez-Marín A, Doñate-Macian P, Gaudet R (2013) What do we know about the transient receptor potential vanilloid 2 (TRPV2) ion channel? FEBS J 280:5471–5487
Cea LA, Riquelme MA, Cisterna BA, Puebla C, Vega JL, Rovegno M et al (2012) Connexin- and pannexin-based channels in normal skeletal muscles and their possible role in muscle atrophy. J Membr Biol 245:423–436
Riquelme MA, Cea LA, Vega JL, Boric MP, Monyer H, Bennett MV et al (2013) The ATP required for potentiation of skeletal muscle contraction is released via pannexin hemichannels. Neuropharmacology 75:594–603
Riquelme MA, Cea LA, Vega JL, Puebla C, Vargas AA, Shoji KF et al (2015) Pannexin channels mediate the acquisition of myogenic commitment in C2C12 reserve cells promoted by P2 receptor activation. Front Cell Dev Biol 3:25 (eCollection)
Araya R, Eckardt D, Maxeiner S, Krüger O, Theis M, Willecke K, Sáez JC (2005) Expression of connexins during differentiation and regeneration of skeletal muscle: functional relevance of connexin43. J Cell Sci 118:27–37
Dahl G, Qiu F, Wang J (2013) The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology 75:583–593
Nakazawa K, Liu M, Inoue K, Ohno Y (1997) Potent inhibition by trivalent cations of ATP-gated channels. Eur J Pharmacol 325:237–243
Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP et al (2006) A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther 319:1376–1385
Burr AR, Molkentin JD (2015) Genetic evidence in the mouse solidifies the calcium hypothesis of myofiber death in muscular dystrophy. Cell Death Differ 22:1402–1412
Bia BL, Cassidy PJ, Young ME, Rafael JA, Leighton B, Davies KE et al (1999) Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J Mol Cell Cardiol 31:1857–1862
Vannucchi MG, Corsani L, Azzena GB, Faussone-Pellegrini MS, Mancinelli R (2004) Functional activity and expression of inducible nitric oxide synthase (iNOS) in muscle of the isolated distal colon of mdx mice. Muscle Nerve 29:795–803
Tidball JG, Albrecht DE, Lokensgard BE, Spencer MJ (1995) Apoptosis precedes necrosis of dystrophin-deficient muscle. J Cell Sci 108:2197–2204
Turpin SM, Lancaster GI, Darby I, Febbraio MA, Watt MJ (2006) Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab 291:E1341–E1350
Percy ME, Andrews DF, Thompson MW (1982) Serum creatine kinase in the detection of Duchenne muscular dystrophy carriers: effects of season and multiple testing. Muscle Nerve 5:58–64
Glesby MJ, Rosenmann E, Nylen EG, Wrogemann K (1988) Serum CK, calcium, magnesium, and oxidative phosphorylation in mdx mouse muscular dystrophy. Muscle Nerve 11:852–856
Manning J, O’Malley D (2015) What has the mdx mouse model of duchenne muscular dystrophy contributed to our understanding of this disease? J Muscle Res Cell Motil 36:155–167
Lagrota-Candido J, Vasconcellos R, Cavalcanti M, Bozza M, Savino W, Quirico-Santos T (2002) Resolution of skeletal muscle inflammation in mdx dystrophic mouse is accompanied by increased immunoglobulin and interferon-gamma production. Int J Exp Pathol 83:121–132
Polfliet MM, Fabriek BO, Daniëls WP, Dijkstra CD, van den Berg TK (2006) The rat macrophage scavenger receptor CD163: expression, regulation and role in inflammatory mediator production. Immunobiology 211:419–425
Pigozzo SR, Da Re L, Romualdi C, Mazzara PG, Galletta E, Fletcher S et al (2013) Revertant fibers in the mdx murine model of Duchenne muscular dystrophy: an age- and muscle-related reappraisal. PLoS One 8:e72147
Hoffman EP, Brown RH Jr, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51:919–928
von Maltzahn J, Euwens C, Willecke K, Söhl G (2004) The novel mouse connexin39 gene is expressed in developing striated muscle. J Cell Sci 117:5381–5392
Iwata Y, Katanosaka Y, Hisamitsu T, Wakabayashi S (2007) Enhanced Na+/H+ exchange activity contributes to the pathogenesis of muscular dystrophy via involvement of P2 receptors. Am J Pathol 171:1576–1587
Cerecedo D, Mondragón R, Cisneros B, Martínez-Pérez F, Martínez-Rojas D, Rendón A (2006) Role of dystrophins and utrophins in platelet adhesion process. Br J Haematol 134:83–91
Blake DJ, Weir A, Newey SE, Davies KE (2002) Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82:291–329
Orellana JA, Díaz E, Schalper KA, Vargas AA, Bennett MV, Sáez JC (2011) Cation permeation through connexin 43 hemichannels is cooperative, competitive and saturable with parameters depending on the permeant species. Biochem Biophys Res Commun 409:603–609
Garré JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Sáez JC et al (2010) FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Natl Acad Sci USA 107:22659–22664
Altamirano F, López JR, Henríquez C, Molinski T, Allen PD, Jaimovich E (2012) Increased resting intracellular calcium modulates NF-κB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes. J Biol Chem 287:20876–20887
Whitehead NP, Pham C, Gervasio OL, Allen DG (2008) N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol 586:2003–2014
Lawler JM (2011) Exacerbation of pathology by oxidative stress in respiratory and locomotor muscles with Duchenne muscular dystrophy. J Physiol 589:2161–2170
Miyatake M, Miike T, Zhao J, Yoshioka K, Uchino M, Usuku G (1989) Possible systemic smooth muscle layer dysfunction due to a deficiency of dystrophin in Duchenne muscular dystrophy. J Neurol Sci 93:11–17
Higuchi I, Niiyama T, Uchida Y, Inose M, Nakagawa M, Arimura K et al (1999) Multiple episodes of thrombosis in a patient with Becker muscular dystrophy with marked expression of utrophin on the muscle cell membrane. Acta Neuropathol 98:313–316
Martin EA, Barresi R, Byrne BJ, Tsimerinov EI, Scott BL, Walker AE et al (2012) Tadalafil alleviates muscle ischemia in patients with Becker muscular dystrophy. Sci Transl Med 4:162ra155
Theis M, de Wit C, Schlaeger TM, Eckardt D, Krüger O, Döring B et al (2001) Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis 29:1–13
Maxeiner S, Dedek K, Janssen-Bienhold U, Ammermüller J, Brune H, Kirsch T et al (2005) Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. J Neurosci 25:566–576
Li S, Czubryt MP, McAnally J, Bassel-Duby R, Richardson JA, Wiebel FF et al (2005) Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specificgene deletion in mice. Proc Natl Acad Sci USA 102:1082–1087
Trebbin AL, Hoey AJ (2009) A novel and simple method for genotyping the mdx mouse using high-resolution melt polymerase chain reaction. Muscle Nerve 39:603–608
Shin JH, Hakim CH, Zhang K, Duan D (2011) Genotyping mdx, mdx3cv, and mdx4cv mice by primer competition polymerase chain reaction. Muscle Nerve 43:283–286
Acknowledgments
We thank Ms. Teresa Vergara and Ms. Paola Fernández for their technical support. We also thank members of the Spanish families, whose participation made this study possible. This work was partially supported by CONICYT/PAI Proyecto de Inserción en la Academia 79140023 (to LAC); Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT): Grant 3130662 (to CP); 1150291 (to JCS), ICM-Economía P09-022-F Centro Interdisciplinario de Neurociencias de Valparaíso (to JCS), grants from the Spanish Ministry of Economy and Competitiveness (Consolider CSD2008-00005 and BFU2013-33821) and the Community of Madrid (Neurotec-P2010/BMD-2460) (to LCB). The research stay of LAC and CP in the Bonn laboratory was supported by a grant of CONICYT and the German Academic Exchange Service (to JCS and KW). Additional work in the Bonn laboratory was funded by the German Research Foundation (Wi 270/33.1 and SFB 645, B2) to KW.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors certify that the experiments comply with the current laws of Chile, where the experiments were performed. All protocols were approved by the Bioethics Committee of the Pontificia Universidad Católica de Chile (Protocol No. 176) in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All efforts were made to minimize animal suffering, reduce the number of animals used, and alternatives to in vivo techniques, if available.
Rights and permissions
About this article
Cite this article
Cea, L.A., Puebla, C., Cisterna, B.A. et al. Fast skeletal myofibers of mdx mouse, model of Duchenne muscular dystrophy, express connexin hemichannels that lead to apoptosis. Cell. Mol. Life Sci. 73, 2583–2599 (2016). https://doi.org/10.1007/s00018-016-2132-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2132-2