Abstract
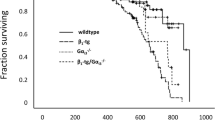
Hypertrophic cardiomyopathy (HCM) is characterized by asymmetric septal hypertrophy and is often caused by mutations in MYBPC3 gene encoding cardiac myosin-binding protein C. In contrast to humans, who are already affected at the heterozygous state, mouse models develop the phenotype mainly at the homozygous state. Evidence from cell culture work suggested that altered proteasome function contributes to the pathogenesis of HCM. Here we tested in two heterozygous Mybpc3-targeted mouse models whether adrenergic stress unmasks a specific cardiac phenotype and proteasome dysfunction. The first model carries a human Mybpc3 mutation (Het-KI), the second is a heterozygous Mybpc3 knock-out (Het-KO). Both models were compared to wild-type (WT) mice. Mice were treated with a combination of isoprenaline and phenylephrine (ISO/PE) or NaCl for 1 week. Whereas ISO/PE induced left ventricular hypertrophy (LVH) with increased posterior wall thickness to a similar extent in all groups, it increased septum thickness only in Het-KI and Het-KO. ISO/PE did not affect the proteasomal chymotrypsin-like activity or β5-subunit protein level in Het-KO or wild-type mice (WT). In contrast, both parameters were markedly lower in Het-KI and negatively correlated with the degree of LVH in Het-KI only. In conclusion, adrenergic stress revealed septal hypertrophy in both heterozygous mouse models of HCM, but proteasome dysfunction only in Het-KI mice, which carry a mutant allele and closely mimic human HCM. This supports the hypothesis that proteasome impairment contributes to the pathophysiology of HCM.






Similar content being viewed by others
References
Ashrafian H, McKenna WJ, Watkins H (2011) Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ Res 109:86–96. doi:109/1/86[pii]10.1161/CIRCRESAHA.111.242974
Bahrudin U, Morisaki H, Morisaki T, Ninomiya H, Higaki K, Nanba E, Igawa O, Takashima S, Mizuta E, Miake J, Yamamoto Y, Shirayoshi Y, Kitakaze M, Carrier L, Hisatome I (2008) Ubiquitin-proteasome system impairment caused by a missense cardiac myosin-binding protein C mutation and associated with cardiac dysfunction in hypertrophic cardiomyopathy. J Mol Biol 384:896–907. doi:10.1016/j.jmb.2008.09.070
Bardswell SC, Cuello F, Rowland AJ, Sadayappan S, Robbins J, Gautel M, Walker JW, Kentish JC, Avkiran M (2010) Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling. J Biol Chem 285:5674–5682. doi:10.1074/jbc.M109.066456
Barefield D, Sadayappan S (2010) Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol 48:866–875. doi:10.1016/j.yjmcc.2009.11.014
Buitrago M, Lorenz K, Maass AH, Oberdorf-Maass S, Keller U, Schmitteckert EM, Ivashchenko Y, Lohse MJ, Engelhardt S (2005) The transcriptional repressor Nab1 is a specific regulator of pathological cardiac hypertrophy. Nat Med 11:837–844. doi:nm1272[pii]10.1038/nm1272
Carrier L, Bonne G, Bahrend E, Yu B, Richard P, Niel F, Hainque B, Cruaud C, Gary F, Labeit S, Bouhour JB, Dubourg O, Desnos M, Hagege AA, Trent RJ, Komajda M, Fiszman M, Schwartz K (1997) Organization and sequence of human cardiac myosin binding protein C gene (MYBPC3) and identification of mutations predicted to produce truncated proteins in familial hypertrophic cardiomyopathy. Circ Res 80:427–434
Carrier L, Knoell R, Vignier N, Keller DI, Bausero P, Prudhon B, Isnard R, Ambroisine ML, Fiszman M, Ross J Jr, Schwartz K, Chien KR (2004) Asymmetric septal hypertrophy in heterozygous cMyBP-C null mice. Cardiovasc Res 63:293–304. doi:10.1016/j.cardiores.2004.04.009
Carrier L, Schlossarek S, Willis MS, Eschenhagen T (2010) The ubiquitin-proteasome system and nonsense-mediated mRNA decay in hypertrophic cardiomyopathy. Cardiovasc Res 85:330–338. doi:10.1093/cvr/cvp247
Cazorla O, Szilagyi S, Vignier N, Salazar G, Kramer E, Vassort G, Carrier L, Lacampagne A (2006) Length and protein kinase A modulations of myocytes in cardiac myosin binding protein C-deficient mice. Cardiovasc Res 69:370–380. doi:10.1016/j.cardiores.2005.11.009
Charron P, Carrier L, Dubourg O, Tesson F, Desnos M, Richard P, Bonne G, Guicheney P, Hainque B, Bouhour JB, Mallet A, Feingold J, Schwartz K, Komajda M (1997) Penetrance of familial hypertrophic cardiomyopathy. Genet Couns 8:107–114
Ciechanover A (2006) The ubiquitin proteolytic system: from a vague idea, through basic mechanisms, and onto human diseases and drug targeting. Neurology 66:S7–S19. doi:10.1212/01.wnl.0000192261.02023.b8
Colson BA, Bekyarova T, Fitzsimons DP, Irving TC, Moss RL (2007) Radial displacement of myosin cross-bridges in mouse myocardium due to ablation of myosin binding protein-C. J Mol Biol 367:36–41. doi:10.1016/j.jmb.2006.12.063
Cuello F, Bardswell SC, Haworth RS, Ehler E, Sadayappan S, Kentish JC, Avkiran M (2011) Novel role for p90 ribosomal S6 kinase in the regulation of cardiac myofilament phosphorylation. J Biol Chem 286:5300–5310. doi:10.1074/jbc.M110.202713
Decker RS, Decker ML, Kulikovskaya I, Nakamura S, Lee DC, Harris K, Klocke FJ, Winegrad S (2005) Myosin-binding protein C phosphorylation, myofibril structure, and contractile function during low-flow ischemia. Circulation 111:906–912. doi:10.1161/01.CIR.0000155609.95618.75
Drews O, Tsukamoto O, Liem D, Streicher J, Wang Y, Ping P (2010) Differential regulation of proteasome function in isoproterenol-induced cardiac hypertrophy. Circ Res 107:1094–1101. doi:10.1161/CIRCRESAHA.110.222364
Eijssen LM, van den Bosch BJ, Vignier N, Lindsey PJ, van den Burg CM, Carrier L, Doevendans PA, van der Vusse GJ, Smeets HJ (2008) Altered myocardial gene expression reveals possible maladaptive processes in heterozygous and homozygous cardiac myosin-binding protein C knockout mice. Genomics 91:52–60. doi:10.1016/j.ygeno.2007.09.005
Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A (2008) Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 29:270–276. doi:10.1093/eurheartj/ehm342
Flavigny J, Souchet M, Sebillon P, Berrebi-Bertrand I, Hainque B, Mallet A, Bril A, Schwartz K, Carrier L (1999) COOH-terminal truncated cardiac myosin-binding protein C mutants resulting from familial hypertrophic cardiomyopathy mutations exhibit altered expression and/or incorporation in fetal rat cardiomyocytes. J Mol Biol 294:443–456. doi:10.1006/jmbi.1999.3276
Fougerousse F, Delezoide AL, Fiszman MY, Schwartz K, Beckmann JS, Carrier L (1998) Cardiac myosin binding protein C gene is specifically expressed in heart during murine and human development. Circ Res 82:130–133
Gautel M, Zuffardi O, Freiburg A, Labeit S (1995) Phosphorylation switches specific for the cardiac isoform of myosin binding protein C: a modulator of cardiac contraction? EMBO J 14:1952–1960
Gautel M, Fürst DO, Cocco A, Schiaffino S (1998) Isoform transitions of the myosin-binding protein C family in developing human and mouse muscles. Lack of isoform transcomplementation in cardiac muscle. Circ Res 82:124–129
Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG (1990) A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell 62:999–1006. doi:0092-8674(90)90274-I[pii]
Geisterfer-Lowrance AA, Christe M, Conner DA, Ingwall JS, Schoen FJ, Seidman CE, Seidman JG (1996) A mouse model of familial hypertrophic cardiomyopathy. Science 272:731–734
Gomes AV, Zong C, Ping P (2006) Protein degradation by the 26S proteasome system in the normal and stressed myocardium. Antioxid Redox Signal 8:1677–1691. doi:10.1089/ars.2006.8.1677
Harris SP, Bartley CR, Hacker TA, McDonald KS, Douglas PS, Greaser ML, Powers PA, Moss RL (2002) Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res 90:594–601
Hedhli N, Depre C (2010) Proteasome inhibitors and cardiac cell growth. Cardiovasc Res 85:321–329. doi:10.1093/cvr/cvp226
Kopito RR (2000) Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 10:524–530. doi:S0962-8924(00)01852-3[pii]
Laporte D, Salin B, Daignan-Fornier B, Sagot I (2008) Reversible cytoplasmic localization of the proteasome in quiescent yeast cells. J Cell Biol 181:737–745. doi:10.1083/jcb.200711154
Lohse MJ, Engelhardt S, Eschenhagen T (2003) What is the role of beta-adrenergic signaling in heart failure? Circ Res 93:896–906. doi:0.1161/01.RES.0000102042.83024.CA93/10/896[pii]
Maass AH, Ikeda K, Oberdorf-Maass S, Maier SK, Leinwand LA (2004) Hypertrophy, fibrosis, and sudden cardiac death in response to pathological stimuli in mice with mutations in cardiac troponin T. Circulation 110:2102–2109. doi:10.1161/01.CIR.0000144460.84795.E3
Maron BJ (2002) Hypertrophic cardiomyopathy: a systematic review. JAMA 287:1308–1320
Marston S, Copeland O, Jacques A, Livesey K, Tsang V, McKenna WJ, Jalilzadeh S, Carballo S, Redwood C, Watkins H (2009) Evidence from human myectomy samples that MYBPC3 mutations cause hypertrophic cardiomyopathy through haploinsufficiency. Circ Res 105:219–222. doi:10.1161/CIRCRESAHA.109.202440
McClellan G, Kulikovskaya I, Winegrad S (2001) Changes in cardiac contractility related to calcium-mediated changes in phosphorylation of myosin-binding protein C. Biophys J 81:1083–1092
McClellan G, Kulikovskaya I, Flavigny J, Carrier L, Winegrad S (2004) Effect of cardiac myosin-binding protein C on stability of the thick filament. J Mol Cell Cardiol 37:823–835. doi:10.1016/j.yjmcc.2004.05.023S0022282804001646[pii]
Mearini G, Schlossarek S, Willis MS, Carrier L (2008) The ubiquitin-proteasome system in cardiac dysfunction. Biochim Biophys Acta 1782:749–763. doi:10.1016/j.bbadis.2008.06.009
Mearini G, Gedicke C, Schlossarek S, Witt CC, Kramer E, Cao P, Gomes MD, Lecker SH, Labeit S, Willis MS, Eschenhagen T, Carrier L (2010) Atrogin-1 and MuRF1 regulate cardiac MyBP-C levels via different mechanisms. Cardiovasc Res 85:357–366. doi:10.1093/cvr/cvp348
Moolman JA, Reith S, Uhl K, Bailey S, Gautel M, Jeschke B, Fischer C, Ochs J, McKenna WJ, Klues H, Vosberg HP (2000) A newly created splice donor site in exon 25 of the MyBP-C gene is responsible for inherited hypertrophic cardiomyopathy with incomplete disease penetrance. Circulation 101:1396–1402
Pohlmann L, Kroger I, Vignier N, Schlossarek S, Kramer E, Coirault C, Sultan KR, El-Armouche A, Winegrad S, Eschenhagen T, Carrier L (2007) Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes. Circ Res 101:928–938. doi:10.1161/CIRCRESAHA.107.158774
Portbury AL, Willis MS, Patterson C (2011) Tearin’ up my heart: proteolysis in the cardiac sarcomere. J Biol Chem 286:9929–9934. doi:10.1074/jbc.R110.170571
Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR, Day SM (2010) Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation 121:997–1004. doi:10.1161/CIRCULATIONAHA.109.904557
Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M (2003) Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations and implications for molecular diagnosis strategy. Circulation 107:2227–2232. doi:10.1161/01.CIR.0000066323.15244.54
Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P (1996) Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 93:841–842
Rottbauer W, Gautel M, Zehelein J, Labeit S, Franz WM, Fischer C, Vollrath B, Mall G, Dietz R, Kubler W, Katus HA (1997) Novel splice donor site mutation in the cardiac myosin-binding protein-C gene in familial hypertrophic cardiomyopathy. Characterization of cardiac transcript and protein. J Clin Invest 100:475–482. doi:10.1172/JCI119555
Saadane N, Alpert L, Chalifour LE (1999) Expression of immediate early genes, GATA-4, and Nkx-2.5 in adrenergic-induced cardiac hypertrophy and during regression in adult mice. Br J Pharmacol 127:1165–1176. doi:10.1038/sj.bjp.0702676
Saadane N, Alpert L, Chalifour LE (2000) Altered molecular response to adrenoreceptor-induced cardiac hypertrophy in Egr-1-deficient mice. Am J Physiol Heart Circ Physiol 278:H796–H805
Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW 2nd, Klevitsky R, Seidman CE, Seidman JG, Robbins J (2005) Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res 97:1156–1163. doi:10.1161/01.RES.0000190605.79013.4d
Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Seidman CE, Seidman JG, Robbins J (2006) Cardiac myosin binding protein c phosphorylation is cardioprotective. Proc Natl Acad Sci USA 103:16918–16923
Sarikas A, Carrier L, Schenke C, Doll D, Flavigny J, Lindenberg KS, Eschenhagen T, Zolk O (2005) Impairment of the ubiquitin-proteasome system by truncated cardiac myosin binding protein C mutants. Cardiovasc Res 66:33–44. doi:10.1016/j.cardiores.2005.12.021
Schlossarek S, Carrier L (2011) The ubiquitin-proteasome system in cardiomyopathies. Curr Opin Cardiol 26:190–195. doi:10.1097/HCO.0b013e32834598fe
Schlossarek S, Mearini G, Carrier L (2011) Cardiac myosin-binding protein C in hypertrophic cardiomyopathy: mechanisms and therapeutic opportunities. J Mol Cell Cardiol 50:613–620. doi:10.1016/j.yjmcc.2011.01.014
Seidman CE, Seidman JG (2011) Identifying sarcomere gene mutations in hypertrophic cardiomyopathy: a personal history. Circ Res 108:743–750. doi:10.1161/CIRCRESAHA.110.223834
Su H, Wang X (2010) The ubiquitin-proteasome system in cardiac proteinopathy: a quality control perspective. Cardiovasc Res 85:253–262. doi:10.1093/cvr/cvp287
van Dijk SJ, Dooijes D, Dos Remedios C, Michels M, Lamers JM, Winegrad S, Schlossarek S, Carrier L, Ten Cate FJ, Stienen GJ, van der Velden J (2009) Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy. Haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation 119:1473–1483. doi:10.1161/CIRCULATIONAHA.108.838672
Vignier N, Schlossarek S, Fraysse B, Mearini G, Kramer E, Pointu H, Mougenot N, Guiard J, Reimer R, Hohenberg H, Schwartz K, Vernet M, Eschenhagen T, Carrier L (2009) Nonsense-mediated mRNA decay and ubiquitin-proteasome system regulate cardiac myosin-binding protein C mutant levels in cardiomyopathic mice. Circ Res 105:239–248. doi:10.1161/CIRCRESAHA.109.201251
Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patterson C (2010) Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ Res 106:463–478. doi:10.1161/CIRCRESAHA.109.208801
Wojcik C, DeMartino GN (2003) Intracellular localization of proteasomes. Int J Biochem Cell Biol 35:579–589
Woodcock EA, Du XJ, Reichelt ME, Graham RM (2008) Cardiac alpha 1-adrenergic drive in pathological remodelling. Cardiovasc Res 77:452–462. doi:10.1093/cvr/cvm078
Acknowledgments
We thank X.J. Wang (University of South Dakota) for the antibody directed against the β5-subunit of the proteasome. This work was supported by the sixth and seventh Framework Programs of the European Union (Marie Curie EXT-014051; Health-F2-2009-241577; Big-Heart project), the Deutsche Forschungsgemeinschaft (FOR-604-CA 618/1-1 and 1-2), and the Leducq Foundation (Research grant Nr. 11, CVR 04).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schlossarek, S., Schuermann, F., Geertz, B. et al. Adrenergic stress reveals septal hypertrophy and proteasome impairment in heterozygous Mybpc3-targeted knock-in mice. J Muscle Res Cell Motil 33, 5–15 (2012). https://doi.org/10.1007/s10974-011-9273-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-011-9273-6