Abstract
We have shown that in murine cardiomyopathy caused by overexpression of the β1-adrenoceptor, Gαi2-deficiency is detrimental. Given the growing evidence for isoform-specific Gαi-functions, we now examined the consequences of Gαi3 deficiency in the same heart-failure model. Mice overexpressing cardiac β1-adrenoceptors with (β1-tg) or without Gαi3-expression (β1-tg/Gαi3−/−) were compared to C57BL/6 wildtypes and global Gαi3-knockouts (Gαi3−/−). The life span of β1-tg mice was significantly shortened but improved when Gαi3 was lacking (95% CI: 592–655 vs. 644–747 days). At 300 days of age, left-ventricular function and survival rate were similar in all groups. At 550 days of age, β1-tg but not β1-tg/Gαi3−/− mice displayed impaired ejection fraction (35 ± 18% vs. 52 ± 16%) compared to wildtype (59 ± 4%) and Gαi3−/− mice (60 ± 5%). Diastolic dysfunction of β1-tg mice was prevented by Gαi3 deficiency, too. The increase of ANP mRNA levels and ventricular fibrosis observed in β1-tg hearts was significantly attenuated in β1-tg/Gαi3−/− mice. Transcript levels of phospholamban, ryanodine receptor 2, and cardiac troponin I were similar in all groups. However, Western blots and phospho-proteomic analyses showed that in β1-tg, but not β1-tg/Gαi3−/− ventricles, phospholamban protein was reduced while its phosphorylation increased. Here, we show that in mice overexpressing the cardiac β1-adrenoceptor, Gαi3 deficiency slows or even prevents cardiomyopathy and increases shortened life span. Previously, we found Gαi2 deficiency to aggravate cardiac dysfunction and mortality in the same heart-failure model. Our findings indicate isoform-specific interventions into Gi-dependent signaling to be promising cardio-protective strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure is a major cause of cardiovascular diseases affecting at least 26 million people worldwide (Savarese and Lund 2017). β-Adrenoceptor antagonists are a cornerstone in the therapy of chronic heart failure because some have been proven to reduce mortality independent of age and gender of the patients (Kotecha et al. 2016, 2017). Guarding the heart from (excessive) β-adrenergic stimulation seems to be cardio-protective mainly by preventing Gs-protein mediated signalling (Baker 2014). Gs-proteins are the cognate interaction partners of β1-adrenoceptors (Xiao et al. 1999; Seyedabadi et al. 2019). Overexpression of β1-adrenoceptors (β1-AR) in murine hearts has been shown to cause dilative cardiomyopathy leading to severe heart failure (Engelhardt et al. 1999, 2001a). Although cardiac overexpression of β2-adrenoceptors (β2-AR) also leads to cardiac failure, a significantly higher level of overexpression is required (Liggett et al. 2000). β2-Adrenoceptors couple to both Gs and Gi proteins (Xiao et al. 1999). Gi proteins are thought to be involved in the protection against excessive β-adrenergic stimulation in heart failure (Brown and Harding 1992; El-Armouche et al. 2003), and Gi-protein-mediated signaling downstream from β2-AR has been shown to be anti-apoptotic (Chesley et al. 2000). Despite the differences between β-adrenoceptor isoforms regarding G-protein coupling, it should be considered that Gi proteins seem to modulate both β1- and β2-adrenergic signalling (Li et al. 2004; Martin et al. 2004; Melsom et al. 2014). It has to be mentioned that not all studies support the idea of Gi proteins mediating the cardio-protective effects of β2-adrenoceptor stimulation (Xiao et al. 2003; Ahmet et al. 2005) or of Gi-protein signaling being cardio-protective in general (Hussain et al. 2013). At least two Gαi-isoforms, Gαi2 and Gαi3, are expressed in the cardiovascular system, which have been shown to interplay (Thompson et al. 2007) and to exhibit redundant but also distinct functions (Gohla et al. 2007; Dizayee et al. 2011; Plummer et al. 2012; Wiege et al. 2012, 2013; Köhler et al. 2014; Wang et al. 2014; Devanathan et al. 2015; Mauriac et al. 2017; Beer-Hammer et al. 2018). Of particular interest, in a murine ischemia–reperfusion model, Köhler et al. showed lack of Gαi2 to worsen cardiac damage while lack of Gαi3 was beneficial (Köhler et al. 2014). Thus, the increased Gαi2-expression observed in failing myocardium might be interpreted as compensatory, while the role of Gαi3 remains unclear (Eschenhagen et al. 1992b; Kompa et al. 1999).
In a previous study, we reported that lack of Gαi2 (Gαi2−/−) had detrimental effects in β1-transgenic (β1-tg) mice (Keller et al. 2015): survival of β1-tg/Gαi2−/− mice was drastically shortened, and these animals showed a significantly impaired cardiac function. This occurred already at an age of about 300 days, i.e., when β1-tg or Gαi2−/− mice were unaffected in this regard. Considering the unknown consequences of functional isoform redundancy between the closely related Gαi2 and Gαi3 proteins on the one hand and isoform-specific, distinct functions on the other hand, we now examined the impact of Gαi3 deficiency on cardiac function of β1-tg mice. In particular, we asked whether the lack of Gαi3 impairs heart function of β1-tg mice, is not detrimental, or may even rescue from β1-AR-induced cardiomyopathy.
We find Gαi3 deficiency to be cardio-protective in terms of slowing down or even preventing the development of β1-AR-induced cardiomyopathy. Together with previous findings, our study indicates isoform-specific targeting of Gαi-protein-mediated signaling to be a promising novel strategy to treat cardiovascular diseases. Parts of the data have already been published as a conference abstract (Schröper et al. 2020).
Methods
Mouse models
Mice with cardiac overexpression of the human β1-AR (β1-tg) have been described earlier (Engelhardt et al. 1999). We had backcrossed these FVB/N-based transgenic mice to a C57BL/6 J background (Keller et al. 2015). In the current study, β1-tg mice were crossbred with mice globally lacking Gαi3 (Gohla et al. 2007), to produce β1-tg Gαi3-deficient mice (β1-tg/Gαi3−/−). Age-matched wildtype and Gαi3-deficient (Gαi3−/−) littermates served as controls. Animals of both sexes were used for our study (sex distribution given in table S1). We kept mice in individually ventilated cages with a 12 h/12 h dark/light cycle and food and water ad libitum. For genotyping, tail or ear clips from 3-week-old mice were processed. Genomic DNA was prepared and genotyping PCR for Gαi3 and the β1-AR was performed as described previously (Dizayee et al. 2011; Keller et al. 2015). Animals were killed by cervical dislocation. Since, in a previous study, cardiac β1-AR overexpression on a C57BL/6 J background by itself had no effect on cardiac function or survival at the age of 300 days (Keller et al. 2015), we chose a second target age to address putative effects of Gαi3 deficiency in β1-tg mice. Based on our own data and the report of another group, we thus additionally analyzed animals at the age of 550 days (Lee et al. 2015; Keller et al. 2015). The responsible federal state authority approved animal breeding, maintenance and experiments (Landesamt fuer Natur-, Umwelt- und Verbraucherschutz Nordrhein-Westfalen; references: 84–02.05.20.12.294, 84–02.05.20.13.060, and 84–02.04.2016.A422). All animal experiments complied with the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Ventricle-to-body-weight ratio
Non-fasting mice were weighed directly before being killed. Immediately after cervical dislocation, we removed the heart, cut the atria and eliminated remaining intraventricular blood. We analyzed mice at an age of 304 ± 7 days and at the second target age of 553 ± 6 days, including mice just examined by echocardiography.
Histology and histomorphometrical analysis of fibrotic area
Only mice at the advanced age (553 ± 3 days) were used for this analysis. Cryo-Sects (6 μm thickness) were obtained from excised hearts frozen in liquid nitrogen, fixed in ice-cold acetone, subsequently immersed in Roti®-Histol for 10 min at room temperature, and transferred to water through descending concentrations of ethanol (100%, 96%, 75%). Staining was performed using a 0.1% solution of Sirius Red F3BA in saturated aqueous solution of picric acid for 45 min at 25 °C. Subsequently, slices were rinsed in 1% acetic acid for 2 min. Sections were dehydrated in ascending concentrations of ethanol (75%, 96%, and 100%, each 1 min) and cleared in two stages in Roti®-Histol, 10 min each. Sections were covered with Roti®-Histokitt mounting medium (Carl Roth, Karlsruhe, Germany) and a glass cover slip. After scanning the Picro Sirius Red sections with the Keyence BZ-9000E microscope, images were taken at mid-ventricular level (× 20 magnification), and interstitial fibrosis was quantified as percentage of total tissue area in the field of view. Planimetry was performed using a Keyence BZ2-Analyser software using hybrid cell count algorithm (Keyence, Osaka, Japan).
Echocardiography
Echocardiography was performed using the high-frequency VisualSonics Vevo® 3100 Imaging System (Fujifilm) with a MX550D transducer (22–55 MHz; axial resolution: 40 µm). Mice were prepared and examined under light inhalation anesthesia with oxygen and 1.5% isoflurane through a nose cap. Chest and upper abdominal hair was shaved, and the mice were placed on a warmed platform to maintain physiological conditions. We monitored ECG, heart rate, core temperature, and respiratory frequency. Systolic parameters were obtained by using the B- and M-Mode in parasternal long and short axis views of the left ventricle. Doppler flow profiles were acquired to estimate the isovolumic relaxation time (IVRT), an indicator of diastolic ventricular function. We evaluated the ultrasound imaging data by working with the software Vevo LAB (Fujifilm). Strain analyses via Speckle Tracking were performed by using the Vevo Strain Software (Fujifilm). Younger mice used for echocardiographic investigation were 303 ± 4, older 551 ± 13 days of age.
Survival analysis
Survival was analyzed by Kaplan–Meier estimation and log-rank test. We defined a priori “spontaneous” death as the event of interest, while being killed for any reason (e.g., organ removal) and survival at the end of the study were considered censored events. Mice used for breading were not included into the survival analysis. Total numbers of mice included in our analysis were 408, 262, 157, and 82 for wildtype, β1-tg, Gαi3−/−, and β1-tg/Gαi3−/−, respectively. Numbers of events of interest during the period of observation were 17, 52, 11, and 13, respectively.
Quantitative real-time PCR
Quantitative real-time PCR (qPCR) was used to reveal the relative ventricular mRNA-expression levels of the Gi isoforms Gαi2 (Gnai2) and Gαi3 (Gnai3), the cardiomyopathy markers atrial natriuretic peptide ANP (Nppa) and brain natriuretic peptide BNP (Nppb), and the phosphorylation targets of protein kinase A (PKA) ryanodine receptor 2 (Ryr2), phospholamban (Pln), and troponin I (Tnni3). Ventricles were stored at − 80 °C until mRNA-isolation. All procedures were performed according to the manufacturer’s protocol (QIAGEN, Hilden, Germany). The RNeasy® Fibrous Tissue Kit (QIAGEN) was used to isolate the mRNA. Quality and quantity of the purified mRNA were controlled by NanoDrop 8000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA). Reverse transcription was done by using the QuantiTect® Reverse Transcription Kit (QIAGEN). All qPCRs were run in triplicates with the ORA™ qPCR Green ROX L Mix, 2X Kit (highQu). Primer pairs for Gnai2, Gnai3, Nppa, Nppb, Ryr2, Pln, and Tnni3 have been reported before (Dizayee et al. 2011; Wiege et al. 2012; Bai et al. 2013; Keller et al. 2015) and are listed in Table S2. The gene encoding 40S ribosomal protein S29 (Rps29) served as a housekeeping gene (Figure S1). The qPCR was initiated with incubation at 95 °C for 15 min. Next, 45 cycles of denaturation were conducted at 95 °C for 15 s. Subsequently, annealing at 60 °C for 25 s, and elongation at 72 °C for 10 s were applied with a transition rate of 20 °C per second. A melting curve analysis was performed at the end to control the product purity at 64 °C for 1 min with a transition rate of 0.1 °C per second. Younger mice used for mRNA-analyses were 301 ± 4, older 554 ± 5 days of age.
Western blot analysis
Liquid-frozen ventricles were homogenized in 500 μl protein lysis buffer (20 mmol/l Tris, pH 8.3; 0.67% SDS; 238 mmol/l 2-mercaptoethanol; 0.2 mmol/l PMSF). Electrophoretic separation of Gαi isoforms was performed in gels containing 6 M urea (Gohla et al. 2007). The proteins were visualized by immunodetection using the following primary antibodies described elsewhere (Beer-Hammer et al. 2018): rabbit anti-Gαi1/i2 (7.2 ng/ml) (Leiss et al. 2020), rabbit anti-Gαi3 (50 ng/ml) (Vega et al. 2020). The protein levels of Gαi2 and Gαi3 were quantified using densitometric analysis software (Image Lab; Bio-Rad, Gräfelfing, Germany) and were normalized to the levels of GAPDH (#2118; Cell Signalling Technology, Frankfurt, Germany) of the same samples. Twenty micrograms protein per lane were loaded. The membranes were first stained with the Gαi2 or Gαi3 antibody, respectively. Membranes were than stripped and stained with the Akt antibody, stripped again, and subsequently stained with the GAPDH antibody to control for equal loading. Ventricles from three animals per genotype were analyzed in three independent experiments. For size orientation, protein standards were loaded (BioRad Precision Plus Protein Standard Dual Colour, and Nippon Genetics BlueStar PLUS Prestained Protein Standard). For the analysis of Akt phosphorylation, we used rabbit antibodies recognizing either total Akt or pAkt only when phosphorylated at Ser473 (#9272 and #9271; Cell Signalling Technology). Phospholamban expression was determined using a mouse monoclonal antibody provided by Badrilla Ltd. (#A010-14). Younger mice used for Western blot analyses were 307 ± 8, older mice 552 ± 8 days of age.
Myocyte preparation for proteomics analyses
We isolated ventricular myocytes from wildtype, β1-tg, Gαi3−/−, and β1-tg/Gαi3−/− mice (n = 3 each; age: 200 ± 56 days). Isolation followed a modified procedure according to Ackers-Johnson et al. (Ackers-Johnson et al. 2016). The chest of anesthetized mice was opened to expose the heart. Descending aorta was cut, and the heart was immediately flushed by injection of 7 ml EDTA buffer into the right ventricle. The heart was removed, and ascending aorta was retrogradely cannulated. Digestion was achieved by sequential injection of 10 ml EDTA buffer, 3 ml perfusion buffer, and 20 ml Liberase buffer (Roche, Liberase TM 0.05 mg/ml) via coronary circulation. Ventricles were then gently pulled into 1-mm pieces using forceps. Cellular dissociation was completed by gentle trituration, followed by addition of 5 ml stop buffer (perfusion buffer containing 5% FBS). Cell suspension was passed through a 100-μm filter, and cells underwent 4 sequential rounds of gravity settling, using perfusion buffer. The supernatant was discarded. The cell pellet in each round was enriched with myocytes and ultimately formed a highly pure myocyte fraction. Cardiomyocyte yields and percentage of viable rod-shaped cells were controlled under an inverse microscope. The final pellet was lysed in buffer (4% SDS in 100 mM Tris/HCl, pH 7.6). Lysates were homogenized, heated at 70 °C for 10 min, and clarified by centrifugation and protein concentrations were determined using the Bio-Rad DC assay. Proteins (1 mg) were precipitated with acetone for 1 h at – 20 °C. The pellet was washed with 80% acetone once and resuspended in 8 M urea buffer (6 M thiourea, 2 M urea in 10 mM HEPES pH 7.5). Proteins were reduced with DTT (10 mM), alkylated with IAA (55 mM) and digested for 3 h with LysC (1:50 enzyme:substrate ratio, Wako chemicals). For further digestion with Trypsin (1:100 enzyme:substrate ratio, Promega), samples were diluted with ammonium bicarbonate buffer (50 mM). For whole proteomics analysis, aliquots of 50 µg were taken and desalted on stage tips. For phospho-proteomic analysis, the remaining 950 µg peptide solution was desalted using SepPak C18 cartridges and dried, and phospho-peptides were enriched using the High-Select TiO2 Phosphopeptide Enrichment Kit (Thermo scientific) following the manufacturer’s instruction.
Proteomics analyses: sample measurement and data processing
Samples were measured on a Q Exactive Plus Hybrid Quadrupol-Orbitrap mass spectrometer (MS) coupled to an EASY-nLC 1000 UHPLC (Thermo Fisher Scientific) and analyzed with a 240 min gradient using Top 10 DDA method. Phospho-proteomic samples were analyzed with a 90 min gradient using Top 10 DDA method. Raw MS data files were analyzed using MaxQuant software (Max Planck Institute of Biochemistry, Martinsried, Germany) (Cox and Mann 2008). We used the Uniprot Mouse database (release November 2019) extended by the human β1-AR (hADRB1) sequence for spectral matching. Default settings were used, and peptides and proteins were identified using a false discovery rate (FDR) of 1%. As variable modification, p(STY) was enabled. To appreciate the biological significance of the differentially phosphorylated proteins, the ingenuity pathway analysis (IPA, QIAGEN, Germany) was used to predict regulated ontology lists, “tox lists” and networks related to cardiovascular function and disease.
Parameters analyzed
With respect to our previous study (Keller et al. 2015), we defined ventricle- to body-weight ratio, conventional echocardiographic parameters of systolic left-ventricular (LV) function (ejection fraction, LV end-systolic volume, LV end-diastolic volume, LV end-systolic length), survival time, mRNA-expression levels of Gnai2, Gnai3, Nppa, Nppb, Ryr2, Pln, and Tnni3, as well as protein expression levels of Gαi2 and Gαi3 as primary parameters. We furthermore included left-ventricular global longitudinal strain (GLS), isovolumic relaxation time (IVRT), and the ratio of E’ and A’ (early and late ventricular relaxation velocity) as additional echocardiographic parameters. GLS, derived from speckle tracking-based echocardiography, is a sensitive parameter for early detection of LV systolic and diastolic dysfunction and has been shown to be an independent predictor of all-cause mortality in (human) heart failure with reduced ejection fraction (Sengeløv et al. 2015; de Lucia et al. 2019). IVRT and the E’ to A’ ratio are sensitive indicators of diastolic function (Alex et al. 2018; Schnelle et al. 2018). Furthermore, fibrotic alterations were quantified as percentage of tissue area in ventricular slices. Western blots were performed to reveal the level of phospholamban expression and the ratio of phosphorylated to total Akt. Levels of protein expression and phosphorylation were furthermore obtained by mass spectrometry done with cardiomyocyte homogenates. Non-primary parameters were obtained and analyzed with an exploratory intention.
Performance of experiments and data analysis
Animals have not explicitly been chosen for a specific experiment or a specific date in a prospective manner. Thus, sequence of investigation was by chance due to availability of an animal at an appropriate age. Sequence of analysis was by chance, too. We did not apply specific methods for randomization of the sequence of experiments or analyses or for blinding of the experimenters. Thus, the analyses were neither specifically blinded nor actively unblinded. Knowledge of the genotype to the investigator may therefore have occurred by chance in individual cases.
Data presentation and statistical analysis
Data are depicted as scatter plots and reported as mean ± standard deviation (SD) in the text. Scatter plots were created using GraphPad Prism and show median and interquartile range or mean ± SD. We performed ANOVAs that (if significant) were followed by Bonferroni-corrected post-tests comparing all groups with respect to most primary parameters (“Performance of experiments and data analysis” section). For mRNA as well as PLN protein expression, we applied Holm-Šídák post-tests referring to age-matched wildtype littermates. In addition, β1-tg and β1-tg/Gαi3−/− mice were compared here. In case of Gnai3 mRNA, wildtype and β1-tg mice were compared by non-parametric Mann–Whitney test. Due to data distribution, log10 values of 2−ΔΔCt were analyzed with respect to ANP and BNP mRNA expression. Due to the small number of samples, we applied the pair wise fixed reallocation randomization test© using the REST-2009® software for mRNA expression in mice at 300 days of age (Pfaffl 2002). The distribution of EF and IVRT values within two groups was compared using a two-sided Fisher’s exact test. Sample size estimation for echocardiography was performed a priori using G*Power 3.1.9.2 software (Heinrich Heine Universität Düsseldorf, Germany). In general, sample sizes were not increased after reviewing the corresponding data, except for qPCR, where the sample size was increased from n = 4 to n = 6–12 per group during a process of manuscript revision. Survival times are reported as mean and 95% confidence interval (CI). Survival analysis was performed by Kaplan–Meier estimation followed by the log-rank test. Throughout, we considered p values < 0.05 as indicating statistically significant differences in confirmatory analyses, i.e., analyses of the primary parameters according to our main scientific questions (see “Parameters analyzed” and “Introduction”). In figures, asterisks then indicate p values below 0.05 (*) and 0.01 (**), respectively. If statistical tests were applied with an exploratory intent (see “Parameters analyzed”), p values are given as numbers down to 0.001, but not indicated by asterisks. Statistical approaches have been specified a priori, except for the analysis of distribution of EF and IVRT values (“Gαi3 deficiency reduces risk of ventricular dysfunction in β1-tg mice” section) that was applied due to the apparent partial overlap of data from β1-tg and β1-tg/Gαi3−/− mice, respectively, and the calculation of Cohen’s d affects sizes that was done using the Psychometrica online effect size calculators (Table S3) (Lenhard and Lenhard 2016). From some animals/tissue probes, we obtained more than one parameter. No particular statistical approach was taken to account for this.
Results
Gαi3 deficiency prolongs survival time in β1-tg animals
In order to get insights into the individual role of the Gαi3 protein in murine cardiomyopathy, mice overexpressing the cardiac β1-adrenoceptor but globally lacking the Gαi3 protein (β1-tg/Gαi3−/−) were compared to β1-adrenoceptor overexpressing (β1-tg), Gαi3-deficient (Gαi3−/−), and wildtype mice. In all mouse lines used, the distribution of genotypes followed Mendel’s rule and that of sex was almost equal (48% male and 52% female). Mice showed no obvious physical phenotype and normal behavior. Deaths occurred suddenly in all groups, at best preceded sporadically by (unspecific) symptoms, e.g., reduced ingestion, striking behavior, or impaired movement.
Survival is a major outcome parameter of heart-failure studies, and in our previous study, we found that Gαi2 deficiency caused a significantly shortened lifetime of β1-tg mice (Keller et al. 2015). In the current study, we followed the survival of the mice up to a maximum age of 880 days. Kaplan-Meyer estimation and log-rank tests revealed that the mean survival time of β1-tg mice was 624 days (95% CI: 592–655), significantly shorter than that of all other genotypes (Fig. 1). Importantly, the concomitant absence of Gαi3 increased the mean life span of β1-tg mice to 696 days (644–747), which was no longer statistically different from the survival time of 789 days for wildtype (739–840) and 742 days for Gαi3−/− mice (693–790), respectively.
Life span of β1-tg mice is significantly shortened compared to wildtype, Gαi3−/−, as well as β1-tg/Gαi3−/− mice. Total numbers of mice included in our analysis were 408, 262, 154, and 82 for wildtype, β1-tg, Gαi3−/−, and β1-tg/Gαi3−/−, respectively. Numbers of spontaneous deaths during the period of observation were 17, 52, 11, and 13, respectively. Kaplan-Meyer estimation and log-rank test were applied. Vertical ticks indicate censored events
In summary, the life span of β1-tg/Gαi3−/− mice was significantly longer than that of β1-tg mice and was not statistically different from that of wildtype littermates, whereas in a previous study, the absence of Gαi2 shortened the life expectancy of β1-tg mice.
Study of animals at an age of 300 days
Given that Gαi2 deficiency already showed adverse effects in 300-day-old β1-tg mice (Keller et al. 2015), we first focused on effects of Gαi3 deficiency at this age.
No cardiac hypertrophy or dysfunction at an age of 300 days
Survival rates of wildtype, Gαi3−/−, β1-tg, and β1-tg/Gαi3−/− were similar at an age of 300 days (see Fig. 1). Ventricle-to-body-weight ratio and echocardiographic parameters of ventricular function were comparable in all groups (Table 1). Only the heart rate was significantly increased in β1-tg mice, which was even more pronounced in β1-tg/Gαi3−/− animals. ANP (Nppa) and especially BNP (Nppb) are useful markers of cardiac hypertrophy and heart failure. Neither Nppa nor Nppb mRNA levels showed statistically significant alterations in β1-tg/Gαi3−/− or Gαi3−/− ventricles compared to wildtypes (Table 2). However, similar to our previous findings (Keller et al. 2015), Nppb mRNA levels were significantly increased in ventricles of β1-tg mice already at this younger age (304 ± 145% of wildtype levels, p < 0.05; Table 2). Expression levels of ryanodine receptor type 2 (Ryr2), phospholamban (Pln), and Troponin I (Tnni3) mRNA were similar in the ventricles of all genotypes (Table 2).
Gnai2 mRNA expression levels were dominant over Gnai3 in wildtype ventricles (data not shown) and similar to wildtype mice in ventricular tissue from β1-tg, β1-tg/Gαi3−/− or Gαi3−/− mice (Table 2). As expected, Gnai3 mRNA expression was not detectable in ventricles of Gαi3−/− and β1-tg/Gαi3−/− mice. Western blot analysis confirmed these findings on the protein level (not shown).
In summary, neither ventricular hypertrophy nor dysfunction was observed in any of the investigated groups at the age of 300 days. In contrast to lack of Gαi2 (Keller et al. 2015), a detrimental effect of Gαi3 deficiency in β1-tg mice at this age is unlikely.
Study of animals at an age of 550 days
In line with increased mortality of β1-tg mice at more advanced ages, we next examined animals at 550 days of age. Effect sizes obtained by comparing wildtype with β1-tg, Gαi3−/− and β1-tg/Gαi3−/− and β1-tg with β1-tg/Gαi3−/− mice at this age can be taken from supplemental Table 3 (Table S3).
Examination of ventricular hypertrophy and fibrosis
At an age of 550 days, β1-tg mice showed ventricular hypertrophy indicated by a statistically significant increase in the mean ventricle-to-body weight ratio compared to wildtype mice (Fig. 2A). No such effect was seen if β1-tg mice were lacking Gαi3. Mice overexpressing the β1-AR developed ventricular fibrosis, which was significantly less pronounced in β1-tg/Gαi3−/− mice (Fig. 2B, C). Fitting to this, mRNA levels of the hypertrophy markers ANP (Nppa) and BNP (Nppb) were significantly increased in β1-tg ventricles, but to a lesser extent when Gαi3 was absent (Nppa: 2578 ± 2323% vs. 705 ± 688%; Nppb: 744 ± 688% vs. 363 ± 260%) (Fig. 2D, E).
Ventricular hypertrophy, fibrosis, and vastly increased hypertrophy markers in β1-tg mice at an age of 550 days. A Ventricle- to body-weight ratios were calculated for 16 wildtype, 21 β1-tg, 13 Gαi3−/−, and 16 β1-tg/Gαi3−/− mice. B, C Sirius Red staining was applied to ventricular cryo-Sects. (3 ventricles from wildtype, β1-tg, and Gαi3−/−, each, and 4 ventricles from β1-tg/Gαi3−/− mice), and interstitial fibrosis was quantified as percentage of total tissue area. Ventricles of β1-tg mice demonstrated an increase in fibrotic area that was significantly attenuated by lack of Gαi3. A, B All groups were compared with each other. C Representative mid-ventricular cardiac sections from wildtype, β1-tg, Gαi3−/−, and β1-tg/Gαi3−/− mice after Sirius Red staining. Scale bars: 100 µm. D, E mRNA expression of ANP (Nppa, D) and BNP (Nppb, E) corresponds to the extent of fibrosis observed in mice overexpressing β1-adrenoceptors. Data from 9 wildtype, 12 β1-tg, 6 Gαi3−/−, and 11 β1-tg/Gαi3−/− mice were obtained in triplicate each. Groups were compared with age-matched wildtypes. In addition, β1-tg and β1-tg/Gαi3−/− mice were compared. Due to data distribution, log10 values of 2−ΔΔCt were analyzed. Scatter plots with median and interquartile range are depicted (A, B, D, and E). * and **: p < 0.05 and p < 0.01 in post-tests following ANOVA (A, D, and E). Fibrotic areas were compared with an exploratory intention, and thus, exact p values are given if < 0.05 (B)
These findings of hypertrophy and fibrosis may be related to ventricular dysfunction in β1-tg mice on the one hand and to protective effects of Gαi3 deficiency on the other. Therefore, we describe below our findings on left ventricular function obtained by echocardiography.
Gα i3 deficiency reduces risk of ventricular dysfunction in β 1 -tg mice
β1-tg mice showed ventricular dysfunction indicated by a statistically significant decrease of the ejection fraction (EF: 35 ± 18%, n = 13) and an increase of the mean LV end-systolic volume, the end-diastolic volume, and the end-systolic length (Fig. 3). In contrast, the EF of β1-tg/Gαi3mice−/− (52 ± 16%, n = 10) was significantly higher than that of β1-tg mice and similar to wildtype (59 ± 4%, n = 8) and Gαi3−/− (60 ± 5%, n = 8). Reduced EF levels were also found in a few β1-tg/Gαi3−/− mice, but significantly less frequently than in β1-tg mice, relative to the 95% CI of age-matched wildtypes (3 out of 10 vs. 12 out of 13, p = 0.003).
Ventricular dysfunction in β1-tg mice at an age of 550 days. As echocardiographic parameters representing systolic function, ejection fraction (A), left-ventricular (LV) end-systolic volume (B), LV end-diastolic volume (C), and LV end-systolic length (D) are shown. Echocardiographic data were obtained from 8 wildtype, 13 β1-tg, 8 Gαi3−/−, and 10 β1-tg/Gαi3−/− mice. Scatter plots with median and interquartile range are depicted. If the ANOVA indicated statistically significant differences, it was followed by Bonferroni-corrected post-tests between all groups. * and **: p < 0.05 and p < 0.01
LV global longitudinal strain (GLS), an independent predictor of all-cause mortality in (human) heart failure with reduced ejection fraction (Sengeløv et al. 2015), was significantly impaired in β1-tg mice in an exploratory analysis (Fig. 4A). In contrast, β1-tg/Gαi3−/− mice did not differ from wildtype and Gαi3−/− mice. Regarding diastolic LV function, we analyzed the isovolumic relaxation time (IVRT). We observed a statistically significant impairment of IVRT in β1-tg mice (Fig. 4B), while the absence of Gαi3 normalized this parameter to wildtype and Gαi3−/− values. Furthermore, the impairment of the E’ to A’ ratio in β1-AR overexpressing mice was no longer observed in β1-tg mice lacking Gαi3 (Fig. 4C).
Exploratory echocardiographic analyses reveal impaired diastolic ventricular function of β1-tg mice at an age of about 550 days. Global longitudinal strain (GLS) (A), isovolumic relaxation time (IVRT) (B), and E’ to A’ ratio (E’/A’) (C) were analyzed as parameters of global (GLS), or diastolic function (IVRT, E’/A’), respectively. Group sizes were 8 for wildtype and Gαi3−/−, 12 (GLS), 11 (E’/A’) and 13 (IVRT) for β1-tg, and 9 (GLS) and 10 (E’/A’ and IVRT) for β1-tg/Gαi3−/− mice, respectively. Scatter plots with median and interquartile range are shown. If the ANOVA indicated statistically significant differences, it was followed by Bonferroni-corrected post-tests between all groups. Analysis was done with an exploratory intention, and thus, exact p values are given if < 0.05
Taken together, Gαi3 deficiency reduced the risk of both systolic and diastolic LV dysfunction in β1-tg mice.
Ventricular expression of G i proteins and Akt
In wildtypes at an age of 550 days, ventricular mRNA levels of Gnai2 transcripts were confirmed to be still dominant over Gnai3 (data not shown). As expected, Gnai3 mRNA was not detectable in ventricles of Gαi3−/− and β1-tg/Gαi3−/− mice (Fig. 5A). Compared to wildtype mice, there was a statistically significant increase of Gnai mRNA in β1-tg ventricles (Gnai2: 397 ± 265%, Gnai3: 196 ± 88%; Fig. 5A, B). There was no statistically significant alteration of Gnai2-mRNA expression in ventricular tissue from β1-tg/Gαi3−/− or Gαi3−/− mice compared to wildtype mice (Fig. 5B). We determined protein expression to corroborate our mRNA data. Except for the absence of Gαi3 in the ventricles of Gαi3−/− and β1-tg/Gαi3−/− mice (Fig. 5C, E), no difference in Gαi3 or Gαi2 expression was found between groups at the protein level (Fig. 5C–F; for uncropped Western blots, see Figure S2).
Gi expression at the mRNA and the protein level. Relative expression of A Gαi3 (Gnai3) and B Gαi2 mRNA (Gnai2) is depicted as 2−ΔΔCt referring to wildtype controls. qPCR data from 550-day-old wildtype (n = 9), β1-tg (n = 12), Gαi3−/− (n = 6), and β1-tg/Gαi3−/− (n = 11) mice were obtained in triplicate each. C, D Representative Western blots of ventricle homogenates isolated from 550-day-old wildtype, β1-tg, Gαi3−/−, and β1-tg/Gαi3−/− mice. To verify antibody specificity, ventricle homogenates from Gαi2-deficient mice were loaded. Gαi3-protein expression (C) is completely absent in Gαi3−/− and β1-tg/Gαi3.−/− ventricles, while not obviously altered in β1-tg ventricles. Gαi2 protein (D) is detectable in ventricles isolated from any genotype. For exemplary full Western blots, see supplemental Figure S2. E, F Statistical analysis of Gαi3 and Gαi2 protein expression patterns using GAPDH as loading control. For Western blot analysis, ventricles from three animals per genotype were analyzed in three independent experiments. Scatter plots with median and interquartile range (A, B) or mean values ± SD are shown (E, F). * and **: p < 0.05 and p < 0.01 in a Mann–Whitney test (A, E) or in post-tests performed if an ANOVA indicated significant differences (B). ANOVA of data on protein expression indicated no difference (F)
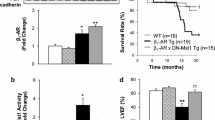
Akt activation has been linked to cardiomyopathy, and studies from other tissues indicated isoform-specific modulation by Gi proteins. We thus analyzed expression of phosphorylated Akt protein. Western blots, however, revealed no obvious differences when comparing pAkt/Akt ratios in ventricles of wildtype, β1-tg, Gαi3−/− and β1-tg/Gαi3−/− mice (Fig. 6). Consistent with this, quantitative proteomics analyses demonstrated no changes in Akt expression or Akt phosphorylation (see the “Proteomics and pathway analyses” section).
A Akt and phosphorylated Akt (pAkt) were detected using specific antibodies in Western blots of ventricle homogenates obtained from 550-day-old wildtype, β1-tg, Gαi3−/−, and β1-tg/Gαi3−/− mice (n = 3 each). B ANOVA did not reveal statistically significant differences of pAkt/Akt ratios (mean values ± SD)
In summary, both Gnai2 and Gnai3 mRNA levels were increased in 550-day-old β1-tg mice while we found no change at the protein level. Akt phosphorylation was not obviously affected by either β1-AR overexpression or Gαi3 deficiency.
Ventricular expression of the PKA targets ryanodine receptor 2, phospholamban, and cardiac troponin I
mRNA levels of the PKA phosphorylation targets ryanodine receptor 2 (Ryr2), phospholamban (Pln), and cardiac troponin I (Tnni3) did not differ between the four genotypes (Figure S3). We furthermore analyzed phospholamban expression by Western blotting (Fig. 7). An ANOVA indicated significant differences (p = 0.036), mainly due to decreased PLN levels in ventricles of β1-tg mice (32 ± 9%) compared to wildtype (100 ± 31%; p = 0.061) and β1-tg/Gαi3−/− mice (107 ± 47%; p = 0.051). Of interest, proteomics analysis furthermore revealed statistically significant alterations of PLN phosphorylation in β1-tg ventricular myocytes, which were not seen in β1-tg/Gαi3−/− mice (see the “Proteomics and pathway analyses” section and Fig. 8C, D).
A Phospholamban (PLN) was detected using a specific antibody in Western blots of ventricle homogenates obtained from 550-day-old wildtype, β1-tg, Gαi3−/−, and β1-tg/Gαi3−/− mice (n = 3 each). B ANOVA indicated statistically significant differences in PLN expression levels normalized to GAPDH (p = 0.036), mainly due to a decrease in β1-tg mice (p = 0.061 vs. wildtype and p = 0.051 vs. β1-tg/Gαi3−/−). Mean values ± SD are depicted
Protein phosphorylation levels in ventricular myocytes (three per genotype) were obtained by mass spectrometry and fed into a so-called ingenuity pathway analysis (IPA), an algorithm-based analysis that uses the QIAGEN knowledge base to identify differences in signaling pathways. A As indicated by − (log p) values, IPA revealed significant differences between β1-tg and β1-tg/Gαi3−/− mice in activation of disease-associated pathways. B Heatmap representing differential phosphorylation of proteins assigned to the “cardiac fibrosis pathway.” Activation Z-scores referring to the respective genotypes compared are color-coded (top row) from blue (“lower activity”) to orange (“higher activity”). An increase in the phosphorylation of a particular protein is shown in red, a decrease in green (lower rows). Gray color indicates comparisons that did not reveal statistical significance (p > 0.05). When comparing β1-tg with wildtype (C), and β1-tg/Gαi3−/− with β1-tg mice (D), the top scoring IPA network indicated differences related to cardiac dysfunction and cardiovascular disease. Red indicates that in the genotype mentioned first, a protein is more phosphorylated relative to the comparator; green stands for reduced phosphorylation. For example, phospholamban (PLN) phosphorylation is increased in β1-tg compared to wildtype myocytes, while it is reduced in β1-tg/Gαi3−/− compared to β1-tg mice. Relationships between proteins (nodes) and heart diseases (cardiac fibrosis, cardiac hypertrophy) are indicated (Tx, toxicity-related (“tox”) lists)
In summary, Western blots indicated reduced PLN expression in ventricles of β1-tg, but not β1-tg/Gαi3−/− mice at the age of 550 days. Ryr2, Pln, and Tnni3 mRNA levels appeared to be unaffected.
Proteomics and pathway analyses
Protein and protein phosphorylation levels in ventricular myocytes from wildtype, β1-tg, Gαi3−/−, and β1-tg/Gαi3−/− mice (n = 3 animals each, age: 200 ± 56 days) were determined by mass spectrometry. We did not detect any change in Akt expression or phosphorylation in agreement with the data from Western blot analysis (cp. Fig. 6). For phospholamban, however, we found a statistically significant increase in phosphorylation at Ser16 (p = 0.007) and Ser17 (p = 0.013) in β1-tg compared to wildtype ventricular myocytes that was not seen in β1-tg/Gαi3−/− myocytes.
For further analysis, we fed the protein phosphorylation data into a so-called ingenuity pathway analysis (IPA). IPA is an algorithm-based analysis that uses the QIAGEN knowledge base to identify differences in signaling pathways. When comparing our β1-tg and β1-tg/Gαi3−/− target genotypes, analyses revealed statistically significant differences associated with multiple cardiac disorders and diseases (Fig. 8A). IPA suggested activation of the predefined protein ontology list “cardiac fibrosis” in β1-tg compared to wildtype ventricles, while activity was reduced in β1-tg/Gαi3−/− compared with data from β1-tg mice (Fig. 8B). To provide further evidence for possible mechanisms, the proteins detected in our probes were mapped to the networks available in the underlying QIAGEN database and then scored using a network score based on p values obtained in Fisher’s exact test. For the sample-specific network that achieved the highest score, β1-tg and β1-tg/Gαi3−/− myocytes showed some differences in phosphorylation of interacting proteins linked to “tox lists” such as “cardiac fibrosis” and “cardiac hypertrophy” (Fig. 8C, D). For example, PLN, RYR2, and calmodulin kinase II (CaMK II) phosphorylation is seen to be increased in β1-tg compared to wildtype myocytes (color-coded red in Fig. 8C), whereas it is lower in β1-tg/Gαi3−/− compared with β1-tg mice (color-coded green in Fig. 8D).
Taken together, proteomics analyses showed that in β1-tg ventricular myocyte phospholamban phosphorylation levels were significantly increased. Pathway and network analyses based upon protein phosphorylation indicated opposite patterns in β1-tg compared to β1-tg/Gαi3−/− mice with respect to cardiac dysfunction and disease. These results are in good agreement with our in vivo data of ventricular dysfunction and our in vitro data such as ventricular fibrosis or increased expression of hypertrophy markers.
Discussion
Given the previously shown detrimental effects of a Gαi2 deficiency in mice with a cardiac overexpression of β1-AR (Keller et al. 2015), we now asked for the role of the closely related Gαi3 isoform in this murine heart-failure model. Since β1-transgenic (β1-tg) mice develop progressively impaired cardiac functions accompanied by a significantly shortened life span, this heart-failure model is suitable to test for effects of an additional Gαi3 deficiency. We wondered how Gαi3 deficiency affects cardiac function and outcome of β1-tg mice, i.e., whether it is detrimental, protective, or has no effect.
Gi proteins in β-AR-mediated heart failure
Our current study revealed that the absence of Gαi3 in β1-AR-overexpressing mice was protective, slowing or even preventing the development of heart failure. In contrast, we previously found that the absence of Gαi2 in β1-tg mice resulted in a distinct heart-failure phenotype even before it was evident in mice overexpressing only the β1-AR (Keller et al. 2015). Thus, the possibility that Gαi3 deficiency mimics the Gαi2-knockout phenotype in the β1-tg model of dilative cardiomyopathy can be excluded. One may hypothesize that the remaining Gαi isoform functionally replaces the missing isoform. Indeed, the absence of one Gαi isoform is often accompanied by upregulation of the remaining one (Wiege et al. 2012; Köhler et al. 2014; Devanathan et al. 2015; Beer-Hammer et al. 2018), although Western blot analyses have been inconsistent regarding an increase of cardiac Gαi2 expression in Gαi3-deficient mice at the protein level (Gohla et al. 2007; Dizayee et al. 2011; Hippe et al. 2013; Köhler et al. 2014). In the current study, we did not see an upregulation of Gαi2 in Gαi3-deficient hearts. However, one should keep in mind that cardiac Gαi2 expression exceeds that of Gαi3 per se. Therefore, we cannot exclude the possibility that Gαi2 contributes by functional substitution even in the presence of unchanged (i.e., “normal”) expression levels. On the other hand, (cardiac) Gαi3 levels might be generally too low to compensate for Gαi2 deficiency. This could explain why we observed adverse effects of Gαi2 deficiency in the previous study, although there was a statistically significant increase in Gαi3-protein expression (Keller et al. 2015). Unfortunately, due to its embryonic lethality, the Gαi2/i3 double knockout mouse model cannot be used to test the assumption that the Gαi isoforms can substitute for each other in the β1-tg mouse model (Gohla et al. 2007). At the mRNA level, expression of both Gαi2 (Gnai2) and Gαi3 (Gnai3) transcripts appeared to be increased in β1-tg ventricles while there was no obvious change at the protein level. In rats treated with isoproterenol, the increase in Gnai mRNA transcript levels was significantly more pronounced than the increase in Gαi protein expression (Mende et al. 1992; Eschenhagen et al. 1992a). Thus, we cannot exclude that we have missed an only slight increase of Gαi expression at the protein level.
Although survival is reduced by cardiac overexpression of β1-adrenoceptors alone, it has been even worse in β1-tg mice lacking Gαi2 (Keller et al. 2015). In contrast, we now find that the life span of β1-tg mice is significantly increased if they lack Gαi3. Although 550-day-old β1-tg mice lacking Gαi3 showed increased cardiac ANP and BNP mRNA levels compared to wildtype littermates, the ANP increase was significantly lower compared to mice only overexpressing the β1-AR. In addition, it appeared to be clearly lower than in Gαi2-deficient β1-tg mice at an age of 300 days as analyzed in our previous study (Keller et al. 2015). Cardiac overexpression of β2-adrenoceptors also leads to cardiac failure, although a significantly higher level of overexpression is required (Liggett et al. 2000). Similar to our recent findings with β1-tg mice, lack of Gαi2 drastically shortened the lifespan of mice with a cardiac overexpression of the β2-AR subtype in another study (Foerster et al. 2003; Keller et al. 2015). Of note, β2-tg mice with a homozygous Gαi2 knockout were virtually non-viable and already heterozygous Gαi2 deficiency reduced life span to a similar extent as did the complete absence of Gαi2 on a background of cardiac β1-AR overexpression. These findings may reflect the role of G-proteins for either β1-AR- or β2-AR-mediated signaling: while Gs proteins are the cognate interaction partners of β1-AR (Xiao et al. 1999; Seyedabadi et al. 2019), it is widely accepted that β2-AR couple to both Gs and Gi proteins (Xiao et al. 1999, 2003). With respect to putative isoform-specific effects of Gi proteins, it should be noted that in a mouse model of ischemia–reperfusion-induced cardiac damage, Köhler et al. also found detrimental effects of Gαi2 deficiency on the one hand while Gαi3 deficiency appeared to be cardioprotective on the other hand (Köhler et al. 2014).
In conclusion, we show that in a mouse model of dilative cardiomyopathy, Gαi3 deficiency is beneficial. In contrast, lack of Gαi2 was clearly detrimental in previous studies, either in the same heart-failure model of β1-AR overexpression, a model of β2-AR overexpression or with ischemia–reperfusion as pathophysiological stimulus (Foerster et al. 2003; Köhler et al. 2014; Keller et al. 2015).
Differences of Gαi2- and Gαi3-dependent effects at the cellular and subcellular level
Data from neutrophils suggest an interesting difference in Gαi2- versus Gαi3-mediated signaling: in a study of Kuwano et al., Gαi2 deficiency led to an increase, but Gαi3 deficiency to a decrease of Akt phosphorylation (Kuwano et al. 2016). Akt has been described to be involved in cardio-protective signaling, while on the other hand, chronic activation of the PI3K/Akt cascade is related to cardiac hypertrophy, and Akt activity was increased in human failing hearts (Haq et al. 2001; Nagoshi et al. 2005). In a previous study, we found no difference between Akt phosphorylation in Gαi2- or Gαi3-deficient mice, neither under basal conditions nor after treating mice with carbachol (Dizayee et al. 2011). The opposing results of our previous study and that of Kuwano et al. may be explained not only by the various tissues analyzed but the different genetic backgrounds (C57/BL6 and 129/Sv, respectively) which have been associated with phenotypic differences in Gi-knockout models (Offermanns 1999; Kuwano et al. 2016). Regarding our previous findings on Akt in mice lacking Gαi2 or Gαi3 (Dizayee et al. 2011), one should bear in mind that carbachol is rather considered a non-pathologic stimulus, and stimulation of muscarinic receptors might be beneficial under pathological conditions, e.g., heart failure (Communal et al. 1999; Olshansky et al. 2008; Lorenz et al. 2009). Although we cannot eventually rule out a change, no obvious differences in ventricular pAkt expression were found in the current study. Given the otherwise pronounced effects on Akt phosphorylation in human heart failure and in heart failure models (Haq et al. 2001; Baba et al. 2003; Miyamoto et al. 2004), it seems at least unlikely that the marked differences in cardiac function and survival in our study can be explained by changes in Akt phosphorylation.
Western blots suggested a reduced PLN expression in β1-tg compared to both wildtype and β1-tg/Gαi3−/− mice. We furthermore used protein expression and phosphorylation data obtained from ventricular myocytes for ingenuity pathway analysis (IPA). IPA has the advantage of a lower risk of bias than manual analysis of the results would have. Our data indicate significant differences between β1-tg and β1-tg/Gαi3−/− mice with respect to intracellular signaling relevant to several cardiac diseases including arrhythmia, heart failure or cardiac fibrosis. Of note, data obtained with ventricular myocytes from Gαi2-deficient mice indicate significant differences to mice lacking Gαi3 in signaling related to cardiac diseases, too (not shown). Proteomics analyses indicated increased phosphorylation of PLN in ventricular myocytes of β1-tg but not β1-tg/Gαi3−/− mice. Our findings on PLN expression and phosphorylation suggest reduced SERCA inhibition in β1-tg hearts. This may be considered compensatory, as SERCA expression and activity are reduced in the setting of heart failure (del Monte and Hajjar 2008). In agreement with this, Engelhardt et al. found that genetic PLN ablation rescued β1-tg mice from heart failure (Engelhardt et al. 2004). It is tempting to speculate that the absence of compensatory PLN changes in β1-tg/Gαi3−/− mice is indicative of cardioprotection by Gαi3 deficiency, as reduced PLN activity is not needed here. In Gαi2-deficient ventricles, our proteomics analysis revealed an increase of PLN phosphorylation similar to that in β1-tg specimens (not shown). This is interesting because in our previous study, Gαi2 deficiency alone already led to reduced life expectancy, but this effect was dramatically more pronounced when these animals also overexpressed the cardiac β1-AR (Keller et al. 2015).
Previously, we found a decreased density of ventricular L-type calcium currents (LTCC) in ventricular cardiomyocytes from Gαi2-deficient mice, while it was increased in Gαi3-deficient cardiomyocytes (Dizayee et al. 2011). Though in other models an increase of ventricular calcium currents led to cardiac damage and dysfunction in the long run (Muth et al. 1999; Nakayama et al. 2007; Beetz et al. 2009), Gαi3 deficiency does not impair cardiac function ((Jain et al. 2001) and this study). β2-Adrenoceptors couple to both Gs and Gi proteins, while Gs proteins are considered the cognate interaction partners of β1-adrenoceptors (Xiao et al. 1999; Seyedabadi et al. 2019). However, β1- and β2-adrenergic signaling seems to be modulated by Gi proteins including mechanisms independent of direct receptor coupling (Li et al. 2004; Martin et al. 2004; Melsom et al. 2014). Thus, it cannot be excluded that the above-mentioned differences between ventricular calcium currents in either Gαi2- or Gαi3-deficient mice also have a role in the development of cardiomyopathy in the β1-tg mouse model.
The data discussed so far do not explain our findings regarding the opposing effects of Gαi2 and Gαi3, but PLN expression and activity as well as ventricular L-type calcium currents should be the subject of further investigations into possible molecular mechanisms underlying the differential effects of Gαi isoforms in cardiomyopathy. Figure 9 and Table 3 summarize results on mechanisms that might contribute to isoform-specific signaling via inhibitory G-proteins in the heart.
Isoform-specific Gαi functions possibly involved in heart disease. 1: Gαi2, but not Gαi3, mediates signal transduction upon M-AChR stimulation and thereby may protect against β1-AR-mediated overstimulation, e.g., with respect to Ca2+ influx via L-type Ca2+ channels (CaV1.2) (Nagata et al. 2000). 2: With increased signal transduction via β2-AR, Gαi2 increases the activity of individual CaV1.2, while Gαi3 inhibits channel activity (Foerster et al. 2003; Klein 2009). 3: The coupling of β2-AR to Gαi3 may be stronger than to Gαi2, e.g., depending on the local membrane charge (Strohman et al. 2019). 4: Under basal conditions, Gαi2 appears to increase CaV1.2-mediated ICaL or to compensate for presumed inhibitory effects of Gαi3 and vice versa ((Dizayee et al. 2011), but: (Nagata et al. 2000)). 5: Gαi2, but not Gαi3, mediates phosphorylation of ERK and may thereby be involved in the stimulation of CaV1.2 (Dizayee et al. 2011). The effect of ERK phosphorylation on heart disease has been described to be either harmful or protective, probably depending on the stimulus (Lorenz et al. 2009; Ruppert et al. 2013). Overall, Gi proteins differentially modulate CaV1.2 and thus ICaL via several isoform-specific mechanisms that depend among other things on the (level of) activity of β1-AR, β2-AR, and/or M-AChR. Alterations in CaV1.2 activity and/or ICaL have been associated with cardiomyopathy and heart failure (6). 7: Gαi2 deficiency led to an increase, Gαi3 deficiency to a decrease of Kir3/GIRK mediated currents (Nobles et al. 2018). Lack of Gαi2 thus might be pro-arrhythmic. Arrhythmia is a major reason of death in heart failure, and rhythm disturbances might cause or aggravate cardiomyopathy (8). “ + ” means stimulation/increase, “ − ” inhibition/decrease. “?” indicates that an interaction, a contribution or a consequence is not clear or fully understood. AC: adenylyl cyclase; β1-AR: β1-adrenoceptor; β2-AR: β2-adrenoceptor; ERK: extracellular signal-regulated kinase; Kir3/GIRK: inward-rectifier potassium channel/G protein-coupled inward-rectifier potassium channel; M-AChR: muscarinic acetylcholine receptor
Limitations of the study
The focus of our study centered on the hypothesis that Gαi3 and Gαi2 have different isoform-specific effects in a mouse model of dilated cardiomyopathy, despite sharing very high amino acid identity. In fact, we found significant functional differences between the two Gαi isoforms. When evaluating these results, however, some methodological peculiarities must be considered that have an impact on the interpretation of the results.
Firstly, the data on qualitatively distinct differences in the effects of Gαi2 (Keller et al. 2015) and Gαi3 deficiency are based on two separate studies in which the gene-deficient mice were each tested against wildtype controls, but not directly against each other. Not least for animal welfare reasons, we were not able to retest a Gαi2-deficient cohort in our current study.
Furthermore, we used global Gαi knockouts in the current and our previous study (Keller et al. 2015). Thus, we cannot exclude the possibility that extra-cardiac effects had an impact on the cardiac phenotype. However, previous studies comparing Gαi2- and Gαi3-deficient mice with their respective wildtype controls showed that, for example, basal heart rate or blood pressure was unchanged (Jain et al. 2001; Albarrán-Juárez et al. 2009). Furthermore, unaltered hypotensive effects following systemic α2-AR stimulation indicated normal circulatory regulation in Gαi2- and Gαi3-deficient mice, respectively (Albarrán-Juárez et al. 2009). We did not obtain catecholamine levels in our study. In a previous study, however, norepinephrine release from atria or brain cortex slices was not altered in Gαi2- or Gαi3-deficient mice (Albarrán-Juárez et al. 2009). Furthermore, given the unchanged basal values of heart rate and blood pressure in the absence of Gαi2 or Gαi3, significant changes in catecholamine levels seem rather unlikely (Jain et al. 2001; Albarrán-Juárez et al. 2009; Keller et al. 2015). Another study revealed the contribution of endogenous catecholamines to the phenotype of β1-tg mice to be negligible, thus arguing against a significant increase in catecholamine levels in this model, too (Engelhardt et al. 2001b).
The mouse model of β1-AR overexpression is a well-established and thoroughly characterized murine heart-failure model, but differs in some features from human heart failure. For example, there is up—instead of down—regulation of β1-AR (Bristow et al. 1986; Engelhardt et al. 1999). However, β1-AR overexpression can be considered as mimicking the chronically increased sympathetic stimulation observed in human heart failure (Engelhardt et al. 1999; Baker 2014). Although the transgenic approach displays a “non-physiologically” high β1-AR expression level, the (over-)expression levels in the C57BL/6-based mice we used here are significantly lower than on the FVB/N background on which the model was originally generated (Keller et al. 2015).
Here, we analyzed cardiac function only under basal conditions. A future study using stressors (e.g., dobutamine), could potentially reveal further differences between Gαi isoforms, e.g., a possibly increased functional reserve in β1-tg mice lacking Gαi3.
Some of our results were supported by proteomics analysis (e.g., with respect to PLN, fibrosis, or hypertrophy). However, it should be noted that this was mainly a screening approach. Nevertheless, the results obtained may point to future studies on the role and molecular mechanisms of Gαi2- and Gαi3-mediated signaling in heart failure.
Conclusion
Gαi3 deficiency has no detrimental effects in a mouse model of dilative cardiomyopathy and even appears to be cardio-protective. Our current and previous results indicate a β1-AR-mediated impairment whose development is oppositely associated with the expression of either Gαi2 or Gαi3. Although the underlying molecular mechanisms remain to be elucidated in further studies, our findings indicate isoform-specific interventions into Gi-dependent signaling pathways (e.g., inhibiting Gαi3) to be promising novel strategies for cardio-protective therapies.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ackers-Johnson M, Li PY, Holmes AP et al (2016) A simplified, Langendorff-free method for concomitant isolation of viable cardiac myocytes and nonmyocytes from the adult mouse heart. Circ Res 119:909–920. https://doi.org/10.1161/CIRCRESAHA.116.309202
Ahmet I, Lakatta EG, Talan MI (2005) Pharmacological stimulation of β2-adrenergic receptors (β2AR) enhances therapeutic effectiveness of β1AR blockade in rodent dilated ischemic cardiomyopathy. Heart Fail Rev 10:289–296. https://doi.org/10.1007/s10741-005-7543-3
Albarrán-Juárez J, Gilsbach R, Piekorz RP et al (2009) Modulation of α2-adrenoceptor functions by heterotrimeric Gαi protein isoforms. J Pharmacol Exp Ther 331:35–44. https://doi.org/10.1124/jpet.109.157230
Alex L, Russo I, Holoborodko V, Frangogiannis NG (2018) Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 315:H934–H949. https://doi.org/10.1152/ajpheart.00238.2018
Baba HA, Stypmann J, Grabellus F et al (2003) Dynamic regulation of MEK/Erks and Akt/GSK-3β in human end-stage heart failure after left ventricular mechanical support: myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc Res 59:390–399. https://doi.org/10.1016/S0008-6363(03)00393-6
Bai Y, Morgan EE, Giovannucci DR et al (2013) Different roles of the cardiac Na + /Ca 2+ -exchanger in ouabain-induced inotropy, cell signaling, and hypertrophy. American Journal of Physiology-Heart and Circulatory Physiology 304:H427–H435. https://doi.org/10.1152/ajpheart.00462.2012
Baker AJ (2014) Adrenergic signaling in heart failure: a balance of toxic and protective effects. Pflugers Arch 466:1139–1150. https://doi.org/10.1007/s00424-014-1491-5
Beer-Hammer S, Lee SC, Mauriac SA et al (2018) Gα i proteins are indispensable for hearing. Cell Physiol Biochem 47:1509–1532. https://doi.org/10.1159/000490867
Beetz N, Hein L, Meszaros J et al (2009) Transgenic simulation of human heart failure-like L-type Ca2+-channels: implications for fibrosis and heart rate in mice. Cardiovasc Res 84:396–406. https://doi.org/10.1093/cvr/cvp251
Bristow MR, Ginsburg R, Umans V et al (1986) β1- and β2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective β1-receptor down-regulation in heart failure. Circ Res 59:297–309. https://doi.org/10.1161/01.RES.59.3.297
Brown LA, Harding SE (1992) The effect of pertussis toxin on β-adrenoceptor responses in isolated cardiac myocytes from noradrenaline-treated guinea-pigs and patients with cardiac failure. Br J Pharmacol 106:115–122. https://doi.org/10.1111/j.1476-5381.1992.tb14302.x
Chesley A, Lundberg MS, Asai T et al (2000) The β2-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3’-kinase. Circ Res 87:1172–1179. https://doi.org/10.1161/01.RES.87.12.1172
Communal C, Singh K, Sawyer DB, Colucci WS (1999) Opposing effects of β1- and β2-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation 100:2210–2212. https://doi.org/10.1161/01.CIR.100.22.2210
Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26:1367–1372. https://doi.org/10.1038/nbt.1511
del Monte F, Hajjar RJ (2008) Intracellular devastation in heart failure. Heart Fail Rev 13:151–162. https://doi.org/10.1007/s10741-007-9071-9
de Lucia C, Wallner M, Eaton DM et al (2019) Echocardiographic strain analysis for the early detection of left ventricular systolic/diastolic dysfunction and dyssynchrony in a mouse model of physiological aging. The Journals of Gerontology: Series A 74:455–461. https://doi.org/10.1093/gerona/gly139
Devanathan V, Hagedorn I, Köhler D et al (2015) Platelet Gi protein Gαi2 is an essential mediator of thrombo-inflammatory organ damage in mice. Proc Natl Acad Sci U S A 112:6491–6496. https://doi.org/10.1073/pnas.1505887112
Dizayee S, Kaestner S, Kuck F et al (2011) Gαi2- and Gαi3-specific regulation of voltage-dependent L-type calcium channels in cardiomyocytes. PLoS ONE 6:e24979. https://doi.org/10.1371/journal.pone.0024979
El-Armouche A, Zolk O, Rau T, Eschenhagen T (2003) Inhibitory G-Proteins and their role in desensitization of the adenylyl cyclase pathway in heart failure. Cardiovasc Res 60:478–487. https://doi.org/10.1016/j.cardiores.2003.09.014
Engelhardt S, Boknik P, Keller U et al (2001a) Early impairment of calcium handling and altered expression of junctin in hearts of mice overexpressing the β 1 − adrenergic receptor. FASEB J 15:1–18. https://doi.org/10.1096/fj.01-0107fje
Engelhardt S, Grimmer Y, Fan GH, Lohse MJ (2001b) Constitutive activity of the human beta(1)-adrenergic receptor in beta(1)-receptor transgenic mice. Mol Pharmacol 60:712–7
Engelhardt S, Hein L, Dyachenkow V et al (2004) Altered calcium handling is critically involved in the cardiotoxic effects of chronic β-adrenergic stimulation. Circulation 109:1154–1160. https://doi.org/10.1161/01.CIR.0000117254.68497.39
Engelhardt S, Hein L, Wiesmann F, Lohse MJ (1999) Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A 96:7059–7064. https://doi.org/10.1073/pnas.96.12.7059
Eschenhagen T, Mende U, Diederich M et al (1992a) Long term beta-adrenoceptor-mediated up-regulation of Gi alpha and G(o) alpha mRNA levels and pertussis toxin-sensitive guanine nucleotide-binding proteins in rat heart. Mol Pharmacol 42:773–783
Eschenhagen T, Mende U, Nose M et al (1992b) Increased messenger RNA level of the inhibitory G protein α subunit Giα-2 in human end-stage heart failure. Circ Res 70:688–696. https://doi.org/10.1161/01.res.70.4.688
Foerster K, Groner F, Matthes J et al (2003) Cardioprotection specific for the G protein Gi2 in chronic adrenergic signaling through 2-adrenoceptors. Proc Natl Acad Sci 100:14475–14480. https://doi.org/10.1073/pnas.1936026100
Gohla A, Klement K, Piekorz RP et al (2007) An obligatory requirement for the heterotrimeric G protein G i3 in the antiautophagic action of insulin in the liver. Proc Natl Acad Sci 104:3003–3008
Haq S, Choukroun G, Lim H et al (2001) Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation 103:670–677. https://doi.org/10.1161/01.CIR.103.5.670
Hippe H, Lüdde M, Schnoes K et al (2013) Competition for G βγ dimers mediates a specific cross-talk between stimulatory and inhibitory G protein a subunits of the adenylyl cyclase in cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol 386:459–469. https://doi.org/10.1007/s00210-013-0876-x
Hussain RI, Aronsen JM, Afzal F et al (2013) The functional activity of inhibitory G protein (Gi) is not increased in failing heart ventricle. J Mol Cell Cardiol 56:129–138. https://doi.org/10.1016/j.yjmcc.2012.11.015
Jain M, Lim CC, Nagata K et al (2001) Targeted inactivation of Gα i does not alter cardiac function or β-adrenergic sensitivity. American Journal of Physiology-Heart and Circulatory Physiology 280:H569–H575. https://doi.org/10.1152/ajpheart.2001.280.2.H569
Keller K, Maass M, Dizayee S et al (2015) Lack of Gαi2 leads to dilative cardiomyopathy and increased mortality in β1-adrenoceptor overexpressing mice. Cardiovasc Res 108:348–356. https://doi.org/10.1093/cvr/cvv235
Klein C (2009) Die Bedeutung des G-proteins Gαi3 für das Schaltverhalten kardialer L-Typ-Calciumkanäle in Kardiomyozyten sowie für die Entwicklung von kardialer Hypertrophie und Insuffizienz von Mäusen bei Überexpression des β2-Adrenorezeptors (Dissertation). Thesis, University of Cologne
Köhler D, Devanathan V, De Franz CBO et al (2014) Gαi2 - and Gαi3-deficient mice display opposite severity of myocardial ischemia reperfusion injury. PLoS ONE 9:3–10. https://doi.org/10.1371/journal.pone.0098325
Kompa AR, Gu XH, Evans BA, Summers RJ (1999) Desensitization of cardiac β-adrenoceptor signaling with heart failure produced by myocardial infarction in the rat. Evidence for the role of Gi but not Gs or phosphorylating proteins. J Mol Cell Cardiol 31:1185–1201. https://doi.org/10.1006/jmcc.1999.0951
Kotecha D, Flather MD, Altman DG et al (2017) Heart rate and rhythm and the benefit of beta-blockers in patients with heart failure. J Am Coll Cardiol 69:2885–2896. https://doi.org/10.1016/j.jacc.2017.04.001
Kotecha D, Manzano L, Krum H et al (2016) Effect of age and sex on efficacy and tolerability of β blockers in patients with heart failure with reduced ejection fraction: Individual patient data meta-analysis. BMJ 353:i1855. https://doi.org/10.1136/bmj.i1855
Kuwano Y, Adler M, Zhang H et al (2016) Gαi2 and Gαi3 differentially regulate arrest from flow and chemotaxis in mouse neutrophils. J Immunol 196:3828–3833. https://doi.org/10.4049/jimmunol.1500532
Lee GJ, Yan L, Vatner DE, Vatner SF (2015) Mst1 inhibition rescues β1-adrenergic cardiomyopathy by reducing myocyte necrosis and non-myocyte apoptosis rather than myocyte apoptosis. Basic Res Cardiol 110:7. https://doi.org/10.1007/s00395-015-0461-1
Leiss V, Schönsiegel A, Gnad T et al (2020) Lack of Gαi2 proteins in adipocytes attenuates diet-induced obesity. Mol Metab 40:101029. https://doi.org/10.1016/j.molmet.2020.101029
Lenhard W, Lenhard A (2016) Computation of effect sizes. Retrieved from: https://www.psychometrica.de/effect_size.html. Psychometrica. https://doi.org/10.13140/RG.2.2.17823.92329. Accessed 05 Oct 2023
Li F, De Godoy M, Rattan S (2004) Role of adenylate and guanylate cyclases in beta1-, beta2-, and beta3-adrenoceptor-mediated relaxation of internal anal sphincter smooth muscle. J Pharmacol Exp Ther 308:1111–1120. https://doi.org/10.1124/JPET.103.060145
Liggett SB, Tepe NM, Lorenz JN et al (2000) Early and delayed consequences of β2-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation 101:1707–1714. https://doi.org/10.1161/01.CIR.101.14.1707
Lorenz K, Schmitt JP, Vidal M, Lohse MJ (2009) Cardiac hypertrophy: targeting Raf/MEK/ERK1/2-signaling. Int J Biochem Cell Biol 41:2351–2355. https://doi.org/10.1016/j.biocel.2009.08.002
Martin NP, Whalen EJ, Zamah MA et al (2004) PKA-mediated phosphorylation of the β1-adrenergic receptor promotes Gs/Gi switching. Cell Signal 16:1397–1403. https://doi.org/10.1016/J.CELLSIG.2004.05.002
Mauriac SA, Hien YE, Bird JE, et al. (2017) Defective Gpsm2/Gαi3 signalling disrupts stereocilia development and growth cone actin dynamics in Chudley-McCullough syndrome. Nat Commun 8:14907. https://doi.org/10.1038/ncomms14907
Melsom CB, Hussain RI, Ørstavik Ø et al (2014) Non-classical regulation of β1- and β2-adrenoceptor-mediated inotropic responses in rat heart ventricle by the G protein Gi. Naunyn Schmiedebergs Arch Pharmacol 387:1177–1186. https://doi.org/10.1007/s00210-014-1036-7
Mende U, Eschenhagen T, Geertz B et al (1992) Isoprenaline-induced increase in the 40/41 kDa pertussis toxin substrates and functional consequences on contractile response in rat heart. Naunyn Schmiedebergs Arch Pharmacol 345:44–50. https://doi.org/10.1007/BF00175468
Miyamoto T, Takeishi Y, Takahashi H et al (2004) Activation of distinct signal transduction pathways in hypertrophied hearts by pressure and volume overload. Basic Res Cardiol 99:328–337. https://doi.org/10.1007/s00395-004-0482-7
Muth JN, Yamaguchi H, Mikala G et al (1999) Cardiac-specific overexpression of the alpha(1) subunit of the L-type voltage-dependent Ca(2+) channel in transgenic mice. Loss of isoproterenol-induced contraction. J Biol Chem 274:21503–21506
Nagata K, Ye C, Jain M et al (2000) Gαi2 but not Gαi3 is required for muscarinic inhibition of contractility and calcium currents in adult cardiomyocytes. Circ Res 87:903–909. https://doi.org/10.1161/01.RES.87.10.903
Nagoshi T, Matsui T, Aoyama T et al (2005) PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Investig 115:2128–2138. https://doi.org/10.1172/JCI23073
Nakayama H, Chen X, Baines CP et al (2007) Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Investig 117:2431–2444. https://doi.org/10.1172/JCI31060
Nobles M, Montaigne D, Sebastian S et al (2018) Differential effects of inhibitory G protein isoforms on G protein-gated inwardly rectifying K + currents in adult murine atria. Am J Physiol Cell Physiol 314:C616–C626. https://doi.org/10.1152/ajpcell.00271.2016
Offermanns S (1999) New insights into the in vivo function of heterotrimeric G-proteins through gene deletion studies. Naunyn Schmiedebergs Arch Pharmacol 360:5–13. https://doi.org/10.1007/s002109900030
Olshansky B, Sabbah HN, Hauptman PJ et al (2008) Parasympathetic nervous system and heart failure pathophysiology and potential implications for therapy. Circulation 118:863–871. https://doi.org/10.1161/CIRCULATIONAHA.107.760405
Pfaffl MW (2002) Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:36e–336. https://doi.org/10.1093/nar/30.9.e36
Plummer NW, Spicher K, Malphurs J et al (2012) Development of the mammalian axial skeleton requires signaling through the Gαi subfamily of heterotrimeric G proteins. Proc Natl Acad Sci U S A 109:21366–21371. https://doi.org/10.1073/pnas.1219810110
Ruppert C, Deiss K, Herrmann S et al (2013) Interference with ERKThr188 phosphorylation impairs pathological but not physiological cardiac hypertrophy. Proc Natl Acad Sci U S A 110:7440–7445. https://doi.org/10.1073/pnas.1221999110
Savarese G, Lund LH (2017) Global public health burden of heart failure. Card Fail Rev 3:7–11. https://doi.org/10.15420/cfr.2016:25:2
Schnelle M, Catibog N, Zhang M et al (2018) Echocardiographic evaluation of diastolic function in mouse models of heart disease. J Mol Cell Cardiol 114:20–28. https://doi.org/10.1016/j.yjmcc.2017.10.006
Schröper T, Mehrkens D, Leiss V et al (2020) Protective effects of Gαi3-deficiency in a mouse model of β1-adrenoceptor mediated cardiomyopathy. Naunyn Schmiedebergs Arch Pharmacol 393:13–13
Sengeløv M, Jørgensen PG, Jensen JS et al (2015) Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 8:1351–1359. https://doi.org/10.1016/j.jcmg.2015.07.013
Seyedabadi M, Hossein M, Albert PR (2019) Pharmacology & Therapeutics Biased signaling of G protein coupled receptors ( GPCRs ): molecular determinants of GPCR / transducer selectivity and therapeutic potential. Pharmacol Ther. https://doi.org/10.1016/j.pharmthera.2019.05.006
Strohman MJ, Maeda S, Hilger D et al (2019) Local membrane charge regulates β2 adrenergic receptor coupling to Gi3. Nat Commun 10:2234. https://doi.org/10.1038/S41467-019-10108-0
Thompson BD, Jin Y, Wu KH et al (2007) Inhibition of Gαi2 activation by Gαi3 in CXCR3-mediated signaling. J Biol Chem 282:9547–9555. https://doi.org/10.1074/jbc.M610931200
Vega SC, Leiss V, Piekorz R et al (2020) Selective protection of murine cerebral Gi/o-proteins from inactivation by parenterally injected pertussis toxin. J Mol Med 98:97–110. https://doi.org/10.1007/s00109-019-01854-1
Wang Z, Dela Cruz R, Ji F et al (2014) Giα proteins exhibit functional differences in the activation of ERK1/2, Akt and mTORC1 by growth factors in normal and breast cancer cells. Cell Commun Signal 12:10. https://doi.org/10.1186/1478-811X-12-10
Wiege K, Le DD, Syed SN et al (2012) Defective macrophage migration in Gαi2 - but not Gαi3 -deficient mice. J Immunol 189:980–987. https://doi.org/10.4049/jimmunol.1200891
Wiege K, Ali SR, Gewecke B et al (2013) Gαi2 is the essential Gαi protein in immune complex − induced lung disease. J Immunol 190:324–333. https://doi.org/10.4049/jimmunol.1201398
Xiao RP, Cheng H, Zhou YY et al (1999) Recent advances in cardiac β2-adrenergic signal transduction. Circ Res 85:1092–1100. https://doi.org/10.1161/01.RES.85.11.1092
Xiao RP, Zhang SJ, Chakir K et al (2003) Enhanced Gi signaling selectively negates β 2-adrenergic receptor (AR)- but not β1-AR-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation 108:1633–1639. https://doi.org/10.1161/01.CIR.0000087595.17277.73
Acknowledgements
We appreciate the excellent technical support by Cora Fried, Sigrid Kirchmann-Hecht, Mourad Ben Said, Carsten Korte, and Simon Grimm. The authors thank Petra Schiller (Institute of Medical Statistics and Bioinformatics, University of Cologne, Germany) for excellent statistical advice.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the graduate program in Pharmacology and Experimental Therapeutics of the University of Cologne and Bayer Schering Pharma [C25 to T.S. and J.M.] and the Intramural Research Program of the NIH [Z01-ES-101643 to L.B.]. The work of B.N. and V.L. was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (NU 53/9–2 & NU 53/13–1). The work of D.M. was supported by DFG grants GRK 2407 (360043781) and TRR 259 (397484323).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by TS, DM, VL, and JM. The first draft of the manuscript was written by JM, and all authors contributed to the successive versions of the manuscript. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
The manuscript does not contain clinical studies or patient data. The responsible federal state authority approved animal breeding, maintenance, and experiments (Landesamt fuer Natur-, Umwelt- und Verbraucherschutz Nordrhein-Westfalen; references: 84–02.05.20.12.294, 84–02.05.20.13.060, and 84–02.04.2016.A422). All animal experiments complied with the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tobias Schröper, Dennis Mehrkens, and Veronika Leiss contributed equally to this work.
Supplementary information
Below is the link to the electronic supplementary material.




Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schröper, T., Mehrkens, D., Leiss, V. et al. Protective effects of Gαi3 deficiency in a murine heart-failure model of β1-adrenoceptor overexpression. Naunyn-Schmiedeberg's Arch Pharmacol 397, 2401–2420 (2024). https://doi.org/10.1007/s00210-023-02751-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02751-8