Abstract
The study addresses the design and optimization of chemical composition and processing routes of new quenching and partitioning medium-Mn alloy using theoretical and experimental approaches. The thermodynamic calculations using Thermo-Calc and JMatPro software were carried out to characterize the influence of Mn, Si and Al contents on cementite formation and precipitation processes. The evolution of individual phases as a function of temperature under thermodynamic equilibrium conditions was estimated. The investigations included the determination of continuous cooling transformation (CCT) and the time–temperature transformation (TTT) diagrams of a model 4Mn alloy. The calculated equilibrium diagrams were compared with the experimental diagrams determined using dilatometric tests. Microstructural observations were carried out to verify the results of dilatometric measurements. The results of thermodynamic calculations and experimental tests showed the moderate agreement. It is related to the inaccuracy of currently available models in the used software and/or non-equilibrium conditions of experimental tests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advanced high-strength steels (AHSS) typically consist of multiphase microstructures, which are optimized by chemical composition and processing parameters [1,2,3,4]. The current research efforts are primarily focused on the newest, third generation AHSS containing 3–10 mass.% Mn [5,6,7,8]. The outstanding strength-to-ductility ratio exhibited by medium-Mn steels is largely attributed to a relatively high amount of metastable retained austenite (10–30 vol.%), which is able to undergo strain-induced martensitic transformation (SIMT) during plastic deformation [9,10,11]. Therefore, the phase composition design and microstructure development of medium-Mn steels focus on adjusting the fraction and stability of retained austenite (RA) [12,13,14].
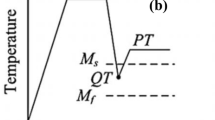
The microstructure of quenching and partitioning (Q&P) steels consists of fine-lath martensite and RA (10–15 vol.%). Such microstructure is produced via austenitization followed by quenching to a temperature between the Ms and Mf in order to obtain a desired fraction of martensite in the microstructure. The steel is then reheated to a higher temperature, where carbon partitioning from the martensite laths into austenite occurs. Sometimes some fraction of carbide-free bainite can be produced. Finally, the steel is quenched to room temperature. Some fraction of austenite not sufficiently enriched in carbon may be transformed into fresh martensite during final quenching [15, 16]. Predicting the final microstructure of steel subjected to Q&P process is a challenging issue. To achieve the desired mechanical properties, precise adjustment of individual microstructural components is necessary, particularly an optimal volume fraction and stability of RA. It is also important to avoid the formation of fresh martensite after final cooling. This is accomplished by carefully determining the time–temperature parameters of Q&P process [17, 18].
Various empirical and physical models have been developed to simulate phase transformation kinetics during annealing and quenching processes. However, there are few works presenting simulations and a thermodynamic approach for designing the chemical composition, kinetics of phase transformations and consequently, the optimization of the Q&P process for medium-manganese steels [19,20,21,22,23,24]. Bouzoni et al. [22] presented a simulation approach to phase transformations and microstructure evolution in low-Si CrMo steel processed through a typical Q&P process. The phase transformations at all processing stages were modeled using kinetic modeling and a phase field model to predict the austenite grain size and subsequent fraction and size of microstructural constituents. Modern approaches to the design of chemical composition and processing parameters often are based on advanced thermodynamic modeling tools, such as Thermo-Calc, Calphad, Dictra or JMatPro software. Onate et al. [23] designed and optimized the chemical composition of a medium-Mn steel alloyed with Si and Nb using the Calphad software. They optimized the Q&P process conditions to improve the stabilization of RA. Behera and Olson [24] reported that the Calphad-based para-equilibrium simulations, enhanced by a temperature-dependent effective stored energy model rooted in a structure of diffusion transformations, have proven to be effective in accurate predicting a C content in austenite in various alloys subjected to different Q&P cycles.
The design of Q&P processing routes requires the detailed thermodynamic analysis to ensure proper conditions for stabilization of 10–15 vol.% of RA and limiting formation of carbides and secondary (fresh) martensite. In this work, the model and experimental approaches were used for designing the chemical composition and processing routes of 4% medium-Mn steel intended for Q&P process.
Material and experimental procedure
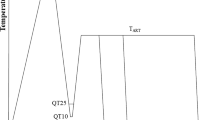
The designing process of the chemical composition of steel is a very complex issue. Hence, using thermodynamic modeling tools such as Thermo-Calc and JMatPro software is an efficient method, which allows to take into account many factors affecting properties of steel. In the present study, the Thermo-Calc (version 2023a with MOBFE7 database) and JMatPro (General Steels Module v11.2 database) software were used for designing the chemical composition of steel, determining its critical temperatures and the kinetics of phase transformation under continuous cooling and isothermal holding conditions. The steel with the optimized chemical composition (the analysis is included in the next chapter) was cast in a vacuum furnace under argon atmosphere into ingots of 100 kg. The cast ingot was austenitized at 1200 °C for 2 h in order to homogenize the chemical composition. Afterward, the material was hot-rolled to a thickness of ~ 22 mm. Hot rolling was performed using the LPS/B semi-industrial line in the temperature range of 1200–900 °C (Łukasiewicz Research Network—Upper Silesian Institute of Technology, Gliwice, Poland). The obtained flat bar was used for further investigations. The investigated steel is intended for a Q&P process. The scheme showing the individual stages of quenching and partitioning processing is presented in Fig. 1.
The results of thermodynamic calculations were experimentally verified using dilatometry. The investigations were carried out using high resolution BÄHR dilatometer DIL 805 A/D. Samples of 4 mm diameter and 10 mm length were machined from the hot-rolled material with a long axis parallel to the rolling direction. The temperature was controlled by a S-type thermocouple welded to the surface of each sample. The tests were performed in vacuum, while helium was used for cooling the samples. The dilatometric data were analyzed according to ASTM A1033-04 standard [25]. First, slow heating of the material at a rate of 2 °C s−1 was performed to determine the Ac1 and Ac3 temperatures and quenching at a rate of 60 °C s−1 to determine Ms temperature [26]. The austenitization was carried out at 1100 °C. Next, different cooling rates in the range from 60 to 0.05 °C s−1 were applied. As the investigations are targeted to the quenching and partitioning process, a critical cooling rate allowing to obtain martensitic structure was determined. A cooling rate of 1 °C s−1 was used for the dilatometric tests including isothermal holding. Isothermal holdings were performed in the range from 440 to 360 °C for 3 h to investigate the kinetics of presumable bainitic transformation. The final cooling was performed at a rate of 1 °C s−1. The martensite-to-austenite ratio in Q&P steels is controlled mainly by the initial quenching carried out in the temperature range between Ms and Mf. According to the literature [1,2,3], the fraction of martensite formed during a quenching step should be in a range of 75–90% to stabilize about 10–20% of retained austenite during a partitioning step to benefit the most from the TRIP effect.
Specimens after the dilatometric measurements were prepared in accordance with standard metallographic procedures. Specimens were cut at one third of their length and embedded in a thermosetting resin. The samples were ground using SiC paper with a grid up to 2000. Then, samples were polished with 3 and 1 µm diamond pastes and finally with the colloidal silica. In order to visualize the microstructure of the steel, the samples were etched in 5% nital. Microstructural analysis was performed using light microscope (Zeiss, Z1m Axio Observer).
Results and discussion
Designing the chemical composition of steel based on thermodynamic calculations
The proper design of chemical composition of steel is crucial for achieving the desired properties. Carbon is an austenite-stabilizing element, which strongly influences the Ms temperature. Carbon interacts with other alloying elements such as manganese forming stable carbides or transition carbides like ε (epsilon) or η (eta) [20, 27, 28]. However, a C content in steel intended for sheets should be limited below 0.2 mass. % due to the negative impact of this element on steel’s weldability [29]. Manganese similarly as carbon is also an austenite-stabilizing element, which significantly increases the hardenability of steel and decreases its Ms temperature. Unfortunately, steel containing elevated Mn contents (above 3 mass. % of Mn) is prone to formation of segregation bands during solidification and hot rolling processes. This phenomenon is related to the relatively low diffusion rate of Mn [30,31,32,33]. Aluminum is a ferrite-stabilizing element and it also increases the Ac3 temperature. Moreover, Al prevents the formation of cementite [34,35,36,37]. Silicon same as Al has the beneficial effect on the reduction of cementite fraction. The results of thermodynamic calculations presented in Fig. 2 show that Si prevents the formation of carbides more effectively than Al. The obtained results showed that the narrowest zone characteristic for the formation of carbides (marked in red) was noted for the alloy containing 0.8 mass% Al and 0.8 mass% Si. Kaar et al. [15] also observed lower effectiveness of Al than Si in terms of suppressing cementite formation in Fe–0.173C–4.46Mn–1.47Si–0.03Al and Fe–0.195C–4.52Mn–0.04Si–1.31Al alloys. They also reported that the substitution of Al by Si has also the beneficial effect on the reduction of Ms temperature and thus the stability of RA. Mahieu et al. [38] observed an increase in Ms temperature by 50 °C, when 1.5 mass% Si was substituted by 1.3 mass% Al. Kim et al. [16] reported that the Si addition stabilizes the austenite during the partitioning step and retards the austenite decomposition process. Moreover, it was also reported in the literature [15, 16] that medium-Mn steels containing Si instead of Al show higher mechanical properties. However, Si has the negative influence on steel sheet’s galvanizing [39]. Niobium controls the grain refinement of steel. The reduction in the grain size has the beneficial effect on strength properties, crack resistance and fatigue strength [40, 41]. The optimized chemical composition of the alloy designed using thermodynamic calculations and the final chemical composition of the melt are presented in Table 1.
Characterization of phase transformation kinetics based on thermodynamic calculations
The theoretical calculations were performed in order to determine phase transformation kinetics and characteristic parameters of the model alloy such as critical temperatures, austenitization temperature and critical cooling rate. Figure 3 shows the equilibrium diagrams of the model alloy. According to the simulations (Fig. 3a), Ac1 and Ac3 temperatures of the alloy were 669 °C and 855 °C, respectively. Therefore, the austenitization temperature of 1100 °C was selected for further calculations and experiments. The results in Fig. 3b show that at the temperatures below 670 °C the formation of particles was possible. The M23C6, M7C3 particles could be expected in the microstructure under equilibrium conditions. However, their fraction will not exceed 3.5 mol.%. The formation of such particles is unfavorable because they trap carbon from the solid solution, which causes that its less amount is available to stabilize RA [42]. Figure 3c shows that stable carbonitrides containing Nb–M(C,N) can be present in the microstructure from a temperature 1246 °C to the room temperature. This means that the hot deformation process should be started above 1200 °C in order to fully utilize the grain refinement effect of Nb microaddition.
The first step of the Q&P process requires quenching of the steel to the temperature between Ms and Mf to obtain a desired fraction of martensite. The formation of ferrite, pearlite or bainite in the microstructure during quenching is undesirable. Therefore, determining the critical cooling rate is necessary. Figure 4 shows the simulations of different cooling rates applied after the austenitization at 1100 °C. The theoretical results show that cooling at 5 °C s−1 was sufficient to obtain the fully martensitic microstructure.
The designing of the time–temperature parameters of Q&P process requires detailed information on the phase transformation kinetics of the model alloy during cooling. The theoretical CCT (Continuous cooling transformation) curves calculated for cooling rates from 110 °C s−1 to 0.0 °C s−1 are shown in Fig. 5. According to the simulations the Ms temperature of the model alloy was 315 °C. The formation of ferrite, pearlite and bainite was shifted to the low cooling rates due to relatively high hardenability of steel caused by the addition of 4 mass.% of Mn. The critical cooling rate allows to obtain the fully martensitic structure after cooling at a rate of about 4.2 °C s−1.
The TTT diagram of the model alloy presented in Fig. 6 shows that the bainitic transformation occurred at a temperature from 460 °C up to Ms temperature. The partitioning step is usually carried out in a temperature range of 400–450 °C [43,44,45]. Therefore, the formation of some bainite fraction in the microstructure should be expected. According to the calculated TTT diagram, the shortest incubation time for bainitic transformation (about 40 s) was noted at a temperature of 400 °C and the bainitic transformation is finished within 1200 s.
Dilatometric and microstructural verification of theoretical calculations
The results obtained using the computational approach were verified using experimental methods. The presented results concern the steel with the chemical composition 0.17C–4.2Mn–0.87Si–0.98Al–0.054Nb obtained for the real melt (Table 1). The characteristic parameters and phase transformation kinetics of the investigated alloy were verified using dilatometry. Figure 7 presents the dilatometric curves obtained during heating and cooling. From this measurement, Ac1 and Ac3 temperatures were determined as 709 °C and 956 °C, respectively (Fig. 7a). Comparing to the data obtained from the theoretical CCT diagram in Fig. 3 (Ac1 = 669 °C and Ac3 = 855 °C) the results showed the moderate agreement. The Ms temperature determined in dilatometric investigations was higher (353 °C) than the calculated one (315 °C)—Fig. 5. The differences between theoretically and experimentally determined Ac1 and Ac3 temperatures were about 40 °C and 101 °C for Ac1 and Ac3 temperatures, respectively. The difference in Ms temperature determined theoretically and the one determined using dilatometry was about 38 °C. The obtained results indicate that at the moment of software development and available databases for novel medium-Mn steels, simulations can only be used to estimate results and be a guide for experimental research.
The investigated steel showed high hardenability due to the elevated Mn content (4 mass.%). The fully martensitic microstructure was obtained in a wide range of cooling rates from 60 °C s−1 to 0.5 °C s−1 (Fig. 8). It is beneficial because air-cooling to the quenching temperature can be applied after hot rolling of steel sheets. This significantly simplifies the technological process and is advantageous for economic reasons. For the cooling rates from 0.1 °C s−1 to 0.05 °C s−1 bainitic transformation occurred during cooling to the room temperature. The results of dilatometric tests were compared with results of microstructural observations. Figure 9 shows the microstructures of investigated steel obtained at different cooling rates from 60 °C s−1 to 0.05 °C s−1. Some fraction of bainite was observed in the microstructure of the sample cooled at a rate of 0.05 °C s−1 (Fig. 9d). At the cooling rates higher than 0.05 °C s−1, the martensitic microstructure was observed. The obtained results are in good agreement with the results obtained using dilatometry (Fig. 8).
Figure 10a shows the experimental TTT diagram of investigated alloy obtained for specimens isothermally annealed within 3 h at different temperatures from 360 °C to 440 °C. The TTT diagram was performed to characterize the kinetics of bainitic transformation. It is especially important in case of Q&P steels because the formation of bainite is sometimes observed during a partitioning step [15, 46, 47]. The duration of the annealing process was selected to enable completing the bainitic transformation. For the 3-h isothermal annealing time no transformations other than the bainitic and martensitic ones were recorded, which is caused by the increased addition of manganese. The lowest incubation time for bainitic transformation was noted for a specimen held at 400 °C (45 s). Moreover, the completion of the bainitic transformation took place the fastest at this temperature after isothermal holding during 3600 s. It means that partitioning at 400 °C may result in a reduced fraction of RA in the microstructure of steel. On the other hand, the tendency for forming undesirable fresh martensite is lower when the formation of bainite in the microstructure was observed during the partitioning step [15]. The obtained experimental results (Fig. 10) were in the moderate agreement with theoretical calculations (Fig. 6). For both approaches the shortest incubation time and completion of the bainitic transformation was noted at 400 °C. The theoretical calculations show the occurrence of ferritic and pearlitic transformations during cooling, while formation of such microstructural constituents was not observed during dilatometric measurements. The differences in the experimental and theoretical results are related to inaccuracy of currently available models in the used software, which do not take into account the reduced Mn diffusion rate in steels with increased manganese contents [48, 49] and/or non-equilibrium conditions of dilatometric tests.
The microstructures obtained for isothermal holdings at different temperatures are shown in Fig. 11. At the highest isothermal holding temperature of 440 °C some small fraction of bainite was observed in the microstructure (Fig. 11a). As the holding temperature decreases, an increased amount of bainite was observed. The obtained microstructures are in good agreement with the results of dilatometric measurements (Fig. 10). Below the Ms the martensitic transformation takes place without the incubation time typical for the bainitic transformation. The bainitic transformation is significantly accelerated after passing the Ms temperature (Fig. 10) due to the formation of prior martensite in the microstructure, which significantly reduces the incubation time and completion time of bainitic transformation. The formation of martensite increases the intensity of bainite nucleation due to strain introduced in the austenite, which provides numerous dislocations being preferential nucleation places [9].
Conclusions
The model and experimental approaches were applied for designing the chemical composition and processing routes of quenching and partitioning 4% medium-Mn steel. The following conclusions can be drawn:
-
The chemical composition of medium-Mn steel, especially the contents of Mn, Si and Al additions play a crucial role in the design of quenching and partitioning processing routes. The results of thermodynamic calculations show that Si prevents the formation of carbides more effectively than Al. The addition of 4 mass. % of Mn provides the high hardenability of steel preventing the formation of ferrite, pearlite or bainite during a quenching step. The optimized alloy contains 0.16C–4Mn–0.8Al–0.8Si–0.04Nb.
-
The critical cooling rate allowing to obtain the fully martensitic microstructure during the quenching step determined in dilatometric tests is 0.5 °C s−1. The critical cooling rate estimated using thermodynamic calculations is significantly higher and equals to 4.2 °C s−1.
-
Both theoretical and experimental results show that some fraction of bainite is formed in the microstructure of the investigated alloy during isothermal heat treatments in a temperature range of 440–360 °C. As the temperature of isothermal holding decreases, the amount of bainite in the structure increases. Moreover, the results of theoretical calculations show that some small fraction of carbides could be also formed at the temperature range typically applied for a partitioning step of Q&P steels.
-
The results of thermodynamic calculations and experimental tests showed the moderate agreement. The largest inconsistencies concern the Ac3 (101 °C) and Ms (38 °C) temperatures and time–temperature range of phase transformations observed during continuous cooling. It is related to the inaccuracy of currently available models in the used software and/or non-equilibrium conditions of dilatometric tests.
References
Zhang X, Gai S, Liu T, Shi Y, Wang Y, Zhou Q, Xing Z, Liu H, Zhai Z. Simultaneous improvement of yield strength and ductility in medium Mn steel by combining gradient structure and TRIP effect. Mater Charact. 2023;197:112650.
Opiela M, Grajcar A. Hot deformation behavior and softening kinetics of Ti-V-B microalloyed steels. Arch Civ Mech Eng. 2012;12:327–33.
Opiela M, Grajcar A. Elaboration of forging conditions on the basis of the precipitation analysis of MX-type phases in microalloyed steels. Arch Civ Mech Eng. 2012;12:427–35.
Liu H, Li J, He B, Liang Z. Effects of soaking temperature on the microstructure and mechanical properties of a medium Mn steel for hot/warm forming. J Mater Res Technol. 2023;26:3496–505.
Lee YK, Han J. Current opinion in medium manganese steel. Mater Sci Technol. 2015;31:843–56.
Morawiec M, Grajcar A, Zalecki W, Garcia-Mateo C, Opiela M. Dilatometric study of phase transformations in 5Mn steel subjected to different heat treatments. Materials. 2020;13:958.
Kim H, Suh DW, Kim NJ. Fe–Al–Mn–C lightweight structural alloys: a review on the microstructures and mechanical properties. STAM. 2013;14:014205.
Grajcar A, Skrzypczyk P, Kozłowska A. Effects of temperature and time of isothermal holding on retained austenite stability in medium-Mn steels. Appl Sci. 2018;8:2156.
Morawiec M, Skowronek A, Kozłowska A, Garcia-Mateo C, Grajcar A. Effect of prior martensite formation on the bainite transformation kinetics in high-strength 3% Mn multiphase steel. J Therm Anal Calorim. 2023;148:1365–71.
Zhang X, Teng R, Liu T, Shi Y, Lv Z, Zhou Q, Wang X, Wang Y, Liu H, Xing Z. Improving strength–ductility synergy in medium Mn steel by combining heterogeneous structure and TRIP effect. Mater Charact. 2022;184:111661.
Grajcar A, Skowronek A, Radwański K. Mechanical behavior and stability of dispersed retained austenite in thermomechanically rolled and isothermally-treated TRIP-aided multiphase steel. Mater Sci Eng A. 2022;830:142300.
Li J, Xu Y, Lu B, Yu Y, Jing Y, Sun W. Improvement of strength-ductility combination in ultra-high-strength medium-Mn Q&P steel by tailoring the characteristics of martensite/ retained austenite constituents. J Mater Res Technol. 2022;18:352–69.
Zou Y, Gao Q, Ding H, Zhang Y, Tang Z. Investigations on austenite stability and martensitic transformation kinetics of a medium Mn steel under different strain states. Int J Plast. 2023;171:103788.
Min J, Hector LG, Zhang L, Lin J, Carsley JE, Sun L. Elevated-temperature mechanical stability and transformation behavior of retained austenite in a quenching and partitioning steel. Mater Sci Eng A. 2016;673:423–9.
Kaar S, Krizan D, Schneider R, Sommitsch C. Impact of Si and Al on microstructural evolution and mechanical properties of lean medium manganese Quenching and Partitioning steels. Steel Res Int. 2020;91:2000181.
Kim B, Sietsma J, Santofimia MJ. The role of silicon in carbon partitioning processes in martensite/austenite microstructures. Mater Des. 2017;127:336–45.
You D, Kim J-K. Partitioning temperature-dependent microstructures and mechanical properties of precipitation-hardened medium-Mn steel. J Mat Res Technol. 2023;27:894–904.
Kantanen P, Anttila S, Karjalainen P, Latypova R, Somani M, Kaijalainen A, Kömi J. Microstructures and mechanical properties of three medium-Mn steels processed via quenching and partitioning as well as austenite reversion heat treatments. Mater Sci Eng A. 2022;847:143341.
Speer JG, Assunção FCR, Matlock DK, Edmonds DV. The “quenching and partitioning” process: background and recent progress. Mat Res. 2005;8:417–23.
Kaar S, Krizan D, Schneider R, Sommitsch C. On competing reactions and austenite stabilization: advanced model for exact microstructural prediction in Q&P steels with elevated Mn-content. Materialia. 2022;26:101584.
Kaar S, Krizan D, Schneider R, Sommitsch C. Tailoring the ductility characteristics of lean-medium Mn quenching and partitioning steels with varying C contents. Steel Res Int. 2023;94:2200966.
Bouzouni M, Gavalas E, Papaefthymiou S. Modeling the microstructure evolution during quenching & partitioning of a conventional CrMo alloy steel. Comput Mater Sci. 2022;206:111265.
Oñate A, Alvarado-Avila MI, Medina C, Villegas C, Ramírez J, Sanhueza JP, Meléndrez M, Rojas D. Characterization of Nb-Si-doped low-carbon steel treated by quenching and partitioning: thermic treatment in two stages supported by computational thermodynamical simulation and controlled sample dimensions. Mater Today Commun. 2023;34:105335.
Behera AK, Olson GB. Nonequilibrium thermodynamic modeling of carbon partitioning in quench and partition (Q&P) steel. Scripta Mater. 2018;147:6–10.
ASTM A1033–04. Standard practice for quantitative measurement and reporting of hypoeutectoid carbon and low-alloy steel phase transformations. ASTM International: West Conshohocken. (2004)
Bała P, Krawczyk J, Lukaszek-Solek A, Pawłowski B, Dziurka R. The analysis of forging of X20Cr13 steel in austenite-ferrite transformation temperature range. In: 2012: Proceedings of the 14th International conference on metal forming.
Xiong J, Liu E, Zhang C, Kong L, Yang H, Zhang X, Wang Y. Tuning mechanical behavior and deformation mechanisms in high-manganese steels via carbon content modification. Mater Sci Eng A. 2023;881:145401.
Bhowmik N, Ghosh S, Mandal S, Haldar A, Chattopadhyay PP, et al. Genesis of plasticity-induced serrated metal flow in medium-Mn steel. Calphad. 2022;77:102425.
Yang J, Dong H, Xia Y, Li P, Hao X, Wang Y, Wu W, Wang B. Carbide precipitates and mechanical properties of medium Mn steel joint with metal inert gas welding. J Mater Sci Technol. 2021;75:48–58.
Singh A, Patra AK, Athreya CN, Sankaran S, Sarma VS. Role of Mn content on processing maps, deformation kinetics, microstructure and texture of as-cast medium Mn (6–10 wt% Mn) steels. Mater Sci Eng A. 2023;884:145500.
Seo EJ, Cho L, De Cooman BC. Kinetics of the partitioning of carbon and substitutional alloying elements during quenching and partitioning (Q&P) processing of medium Mn steel. Acta Mater. 2016;107:354–65.
Lee H, Jo MC, Sohn SS, Zargaran A, Ryu JH, Kim NJ, Lee S, et al. Novel medium-Mn (austenite + martensite) duplex hot-rolled steel achieving 1.6 GPa strength with 20% ductility by Mn-segregation-induced TRIP mechanism. Acta Mater. 2018;147:247–60.
Allam T, Bleck W, Klinkenberg C, Kintscher B, Krupp U, Rudnizki J. The continuous casting behavior of medium manganese steels. J Mater Res Technol. 2021;15:292–305.
Injeti VSY, Li ZC, Yu B, Misra RDK, Cai ZH, Ding H. Macro to nanoscale deformation of transformation-induced plasticity steels: impact of aluminum on the microstructure and deformation behavior. J Mater Sci Technol. 2018;34:745–55.
Yu W, Qian L, Peng X, Wang T, Li K, Wei C, Chen Z, Zhang F, Meng J, et al. Roles of Al in enhancing the thermal stability of reverted austenite and mechanical properties of a medium-Mn TRIP steel containing 2.7 Mn. Mater Sci Technol. 2023;167:119–36.
Ren J, Zhang L, Li Z, Chen J, Zhang W, Wu S, Liu Z, et al. Fe-Mn-Al-C high-entropy steels with superior mechanical properties at 4.2 K. Mater Des. 2023;228:111840.
Emo J, Maugis P, Perlade A. Austenite growth and stability in medium Mn, medium Al Fe-C-Mn-Al steels. Comput Mater Sci. 2016;125:206–17.
Mahieu J, Maki J, De Cooman BC, Claessens S. Phase transformation and mechanical properties of Si-free CMnAl transformation-induced plasticity-aided steel. Metall Mater Trans A. 2002;33:2573–80.
Wallner M, Schneider R, Steineder K, Sommitsch C. Effect of Si and Al on microstructure-property relationship of Q&P-steels during galvannealing. J Mater Sci Technol. 2021;37:182–9.
Varanasi RS, Gault B, Ponge D. Effect of Nb micro-alloying on austenite nucleation and growth in a medium manganese steel during intercritical annealing. Acta Mater. 2022;229:117786.
Zhang D, Liu G, Zhang K, Sun X, Liang X, Yong Q. Effect of Nb microalloying on microstructure evolution and mechanical properties in low carbon medium manganese steel. Mater Sci Eng A. 2021;824:141813.
Mueller JJ, Hu X, Sun X, Ren Y, Choi K, Barker E, Speer JG, Matlock DK, De Moor E. Austenite formation and cementite dissolution during intercritical annealing of a medium-manganese steel from a martensitic condition. Mater Des. 2021;203:109598.
De Moor E, Speer JG, Matlock DK, Kwak JH, Lee SB. Quenching and partitioning of CMnSi steels containing elevated manganese levels. Steel Res Int. 2012;83:322–7.
De Knijf D, Petrov RH, Föjer C, Kestens LAI. Effect of fresh martensite on the stability of retained austenite in quenching and partitioning steel. Mater Sci Eng A. 2014;615:107–15.
Ebner S, Suppan C, Schnitzer R, Hofer C. Microstructure and mechanical properties of a low C steel subjected to bainitic or quenching and partitioning heat treatments. Mater Sci Eng A. 2018;735:1–9.
Zhang J, Dai Z, Zeng L, Zuo X, Wan J, Rong Y, Chen N, Lu J, Chen H. Revealing carbide precipitation effects and their mechanisms during quenching-partitioning-tempering of a high carbon steel: experiments and modeling. Acta Mater. 2021;217:117176.
Celada-Casero C, Kwakernaak C, Sietsma J, Santofimia MJ. The influence of the austenite grain size on the microstructural development during quenching and partitioning processing of a low-carbon steel. Mater Des. 2019;178:107847.
Du PJ, Yang DP, Bai MK, Xiong XC, Wu D, Wang GD, Yi HL. Austenite stabilization by two step partitioning of manganese and carbon in a Mn-TRIP steel. J Mater Sci Technol. 2019;35:2084–91.
De Moor E, Matlock DK, Speer JG, Merwin MJ. Austenite stabilization through manganese enrichment. Scripta Mater. 2011;64:185–8.
Funding
The research leading to these results has received funding from the Norway Grants 2014–2021 via the National Centre for Research and Development, grant no. NOR/SGS/INNOQPTECHNOL/0177/2020-00. EEA Grants/Norway Grants, NOR/SGS/INNOQPTECHNOL/0177/2020-00.
Author information
Authors and Affiliations
Contributions
[Aleksandra Kozłowska] and [Adam Grajcar] contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Adam Skowronek], [Aleksandra Kozłowska] and [Barbara Grzegorczyk]. The first draft of the manuscript was written by [Aleksandra Kozłowska] and [Barbara Grzegorczyk]. Initial review was caried out by [Adam Grajcar] and [Adam Skowronek]. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kozłowska, A., Skowronek, A., Grzegorczyk, B. et al. Thermodynamic approach for designing processing routes of 4Mn quenching and partitioning steel. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13577-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13577-8