Abstract
Sartans, also known as angiotensin receptor blockers, comprise a category of antihypertensive medications designed to inhibit the actions of angiotensin II (Ang II) in the body, ultimately reducing blood pressure levels. This class of compounds is derived from 2-(1-benzyl-1H-imidazol-5-yl)-acetic acid, with its origin characterized by an imidazole core that underwent various substitutions at specific positions within the heterocyclic nucleus. We investigated the behavior of Losartan, Valsartan and Irbesartan and their compatibility with various excipients used in pharmaceutical tablet formulations by FTIR spectroscopic studies, thermal behavior by thermogravimetry and differential scanning calorimetry. The aim of the study was to determine the excipients to be used in pharmaceutical formulations containing drugs from the class of sartans as active ingredients. Our study concludes by recommending precautionary measures in elaborating new solid formulations containing lactose in the case of Losartan.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Angiotensin II is the bioactive component of the renin-angiotensin system and plays a central role in numerous physiological effects in the human body [1]. This potent molecule is produced by the enzymatic conversion of angiotensin I, which is facilitated by angiotensin-converting enzyme (ACE, kinase II).
Angiotensin II exerts a significant influence on blood pressure regulation, influencing aldosterone secretion and overall homeostasis [2].
Sartans, also called angiotensin receptor blockers (ARBs) or angiotensin receptor antagonists (AT1), belong to a group of antihypertensive drugs that are designed to reduce blood pressure by inhibiting the action of the hormone angiotensin II (Ang II) in the body [3, 4]. Structurally, sartans closely resemble Ang II and act as inhibitors by binding to its receptors.
These compounds are derived from 2-(1-benzyl-1H-imidazol-5-yl)-acetic acid, with their original composition having an imidazole core that undergoes various substitutions at specific positions within the heterocyclic nucleus [5]. Sartans are widely used in clinical practice, primarily for the treatment of conditions such as mild to moderate hypertension, chronic heart failure, prevention of secondary stroke, and diabetic nephropathy [6, 7].
Several methods have been highlighted in the literature to characterize these drugs, including FTIR, HPLC, and UV–VIS. Recent studies have shifted the focus to the investigation of potentially carcinogenic impurities in sartans using the LC–MS method.
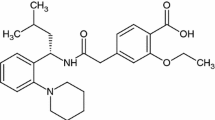
Irbesartan (Irbe) is classified as an orally active angiotensin II type 1 receptor antagonist, commonly known as an angiotensin receptor blocker (ARB). Its pharmacological properties clearly distinguish it from other drugs in the same class, as it has high bioavailability, and offers a prolonged duration of action. Irbe is recommended for patients with renal insufficiency. The drug has shown remarkable efficacy in patients with left ventricular hypertrophy and congestive heart failure, regardless of the stage of renal disease, and is indicated for the treatment of hypertension and renal insufficiency in patients with type 2 diabetes (T2D) and hypertension [8].
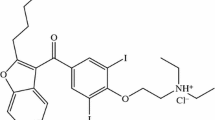
Losartan (Los) is classified as an angiotensin II receptor antagonist (ARAII) and exerts its antihypertensive effect mainly by selectively blocking AT1 receptors, resulting in a reduction in the pressor effect (increasing blood pressure) of angiotensin II. It is also known for its role in reducing the risk of stroke in people with left ventricular hypertrophy and is used in the treatment of diabetic nephropathy (kidney disease associated with diabetes), as well as for its potential benefits associated with myocardial infarction (heart attack) [9].
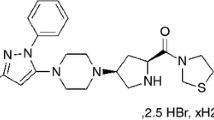
Valsartan (Val) not only has an antihypertensive effect but has been shown to reduce the combined endpoint of mortality and morbidity and produce a remarkable reduction in proteinuria (excess protein in the urine) in people with type II diabetes and heart failure. Compared with amlodipine, it also delays the progression of diabetes in people with reduced glucose tolerance. This suggests that Val may have additional uses beyond its primary use as an antihypertensive drug [10].
To effectively develop a new pharmaceutical formulation, one of the fundamental considerations at an early stage is to evaluate of the compatibility between the active substance and the excipients used in the formulation [11].
Excipients are substances that are incorporated into the final dosage form alongside the active pharmaceutical ingredient and are recognized as having therapeutic benefits, such as improving solubility and permeability, reducing viscosity, and optimizing absorption, but may also have potential interactions [12].
The rationale for selecting the excipients tested is based on their different classifications and specific functions within solid formulations. In addition, it is important to understand the potential interactions, or lack thereof, between the active pharmaceutical ingredient and these excipients. Such understanding is essential because it can significantly affect the stability and content of the active pharmaceutical ingredient.
In this study, several excipients such as starch, hydroxyethyl cellulose, magnesium stearate, lactose, mannitol, talc, silicon dioxide, and polyvinylpyrrolidone were used with different objectives. The main goal was to investigate the potential thermally induced interactions between these excipients and these active substances from the class of sartans, which contain in their structure different functional groups that are very important for the biological activity.
Starch is a versatile excipient used mainly in solid oral dosage forms. Its multifunctional role includes acting as a binder, diluent and disintegrante. Hydroxyethyl cellulose is a nonionic, water-soluble polymer widely used in pharmaceutical formulations as a coating agent, suspending agent, tablet binder, thickener, and viscosity enhancer [13]. Magnesium stearate is mainly used as a lubricant in the manufacture of capsules and tablets.
Lactose is an excipient commonly used as a diluent in tablet and capsule formulations.
In pharmaceutical preparations, mannitol is mainly used as a diluent, plasticizer, sweetener, therapeutic agent, and tonic in tablet formulations because it is non-hygroscopic, which makes it suitable for moisture-sensitive active ingredients. Talc has always been a common choice in oral solid pharmaceutical formulations, especially as a lubricant and diluent, as a dissolution retarder in the development of controlled release products, as a component of powder coatings for extended release granules, as an adsorbent, anti-caking agent, and as a glidant in various pharmaceutical processes. Silica is one of the most used pharmaceutical excipients as a carrier for liquids and semi-liquids or as a free-flowing agent in powder products. It plays an important role in maintaining the desired consistency of pastes and ointments, while preventing the separation of various ingredients. It is an incredibly versatile material with numerous applications in both the pharmaceutical and cosmetic industries, including as an anti-caking agent, slip agent and absorbent especially for hygroscopic or clumping substances, enhancing their flow. Polyvinylpyrrolidone is a biodegradable polymer that is inert, resistant to temperature changes, and stable at various pH values. It plays a crucial role in the encapsulation of hydrophilic and lipophilic drugs as a binder, coating agent, suspending agent, solubilizer and stabilizer. The non-toxicity and biocompatibility of polyvinylpyrrolidone in pharmaceuticals make it a versatile excipient suitable for a wide range of applications [13].
The study of the thermal behavior of pure active substances in binary mixtures with excipients and subsequently in pharmaceutical formulations is of great importance in the study of drugs. In the case of pharmaceutical formulations, the established term is most often correlated with the partial or total decomposition of the pure active substance in the respective pharmaceutical formulation. The decomposition of the active substance due to chemical processes or even physical transitions (polymorphism changes) in the presence of excipients argues the validity of that formulation [14]. Thus, an appropriate choice of excipients can lead to obtaining formulations with a longer shelf life and much better stability, because the role of excipients can also be a stabilizer.
In the case of the present study, the samples were initially investigated by universal total reflection Fourier transform infrared spectroscopy (UATR-FTIR spectroscopy) to provide data to confirm the purity of the active substances and to obtain information about the compatibility of the active substance with the excipients that were used within future solid formulations in environmental conditions. These results were later supplemented with a search for the interactions between the components during heat treatment by thermoanalytical techniques. All these studies led to the validation of excipients that can be used in future solid formulations.
Materials and methods
The Irbesartan standard (Irbe) was obtained from Sigma-Aldrich (LOT: LRAA2751). It is a product of the USA. The Losartan (Los) standard was purchased from STADA Hemofarm (LOT: C5082-16–015). The Valsartan (Val) standard was also procured from STADA Hemofarm (LOT: 00231806 (Fig. 1). All active substances were stored in a refrigerator, shielded from light sources. Excipients used were: Polyvinylpyrrolidone (PVP) M.W. 4000 powder sold by CALBIOCHEM, Merck, Darmstadt, Germania (Lot: BBCV7638, CAS: 9003–39-8), talc Luzenac Pharma, Italy, CAS: 7890–25-7, silicon dioxide (SiO2) from Aerosil 200 Evonik Degussa, Germany, (Manitol) from Merck, Germany, (MgSTSZB) Magnesium Stearate, Sigma-Aldrich, Germany, purity according to the European Pharmacopoeia, Lot #SZBF2590V, CAS 557–04-0, MW 591.24 g mol-1, (LactSLBK) α-Lactose monohydrate, Sigma Life Science, Germany, purity ≥ 99%, Lot #SLBK4809V, CAS 5989–81-1, MW 360.31 g mol-1., Lactose Sigma-Aldrich, Darmstadt, Germany, magnesium stearate (MgSt) purchased from Merck, Germany, Hydroxyethyl cellulose (HEC) from Sigma-Aldrich, Darmstadt, Germany and Starch from Sigma-Aldrich.
These excipients were selected because they are commonly used in pharmaceutical tablet formulations. Several excipients were selected because the active ingredients have different functional groups that can be affected and inactivated by some of these excipients, and therefore there is a possibility that some excipients may be incompatible with this class of drugs.
The Irbe + excipient, Los + excipient and Val + excipient binary mixtures consist of equal masses of each active substance and each excipient and were prepared in a 1:1 mass ratio to maximize the possibility of interactions. All samples, both the individual active substances and the binary mixture were investigated by FTIR, thermogravimetric analysis, and DSC.
FTIR spectroscopy
FTIR-U-ATR spectroscopy data were gathered utilizing a SPECTRUM 100, Perkin Elmer Spectrometer, employing the Universal Attenuated Total Reflectance (U-ATR) technique. The data collection process involved performing 8 consecutive recordings at a resolution of 4 cm−1, covering the spectral range from 4000 to 650 cm−1.
Thermogravimetric analysis (TGA)
The thermal analysis was conducted using an aluminum crucible on TGA/DSC 3 + , Mettler-Toledo GmbH, 8606 Greifensee, Switzerland. The analyses were carried out under a dynamic air atmosphere, oxidative conditions with synthetic air 5.0 from Linde Gas with a flow rate of 20 mL min−1, temperature range 25–400 °C at a heating rate of 10 °C min−1. All samples have a mass around 5 mg. This comprehensive analysis was performed on Irbesartan (Irbe), Losartan (Los), Valsartan (Val), as well as on moist binary mixtures.
Differential scanning calorimetry (DSC)
It was performed using a DSC 3 + , Mettler-Toledo GmbH, 8606 Greifensee, Switzerland, in a temperature range 25–400 °C, nitrogen atmosphere, in aluminum crucibles.
Results and discussion
FTIR analysis
FTIR-UATR analysis of the active substances
The spectrum of each active substance was investigated to compare it with the spectra of the binary mixture studied. In particular, the significant vibrations of the active substances in the spectra of the binary mixtures were followed.
Knowing that the active substances are part of the sartan class, their constituent fragments can be highlighted by the appearance of some characteristic peaks in the FTIR spectra. Thus, as shown in Fig. 2, the diphenyl moiety can be highlighted through the peaks for the stretching vibrations of –C=C– and = C–H bonds from the benzene ring in the region of 1300–1500 cm−1 (blue rectangle). As a constituent part, the tetrazole ring confirms its presence through two peaks: the stretching vibration of νN=N from ≈1550 cm−1 and the peaks for νN–N in the area 1050–1140 cm−1 (yellow rectangle). The imidazole core is responsible for the appearance of the peak for the stretching vibration of the C–N bond at ≈ 1250 cm−1, whereas the butyl fragment attached to this core contributes to the appearance of the peak for νC–H at 2960 cm−1. An interesting fact is that in the fingerprint region, all active substances present a very intense peak at 757 cm−1, which according to the literature can be attributed to γN-H [15,16,17].
As expected, due to the presence of the ketone carbonyl, only in the case of the Irbe and Val sample can be seen the νC=O peak, at 1730 cm−1, this being much more intense in the case of the Val sample due to the presence of two C=O groups. Moreover, since an N atom is found in the immediate vicinity of the carbonyl, an intramolecular amide fragment is formed, which directly leads to the appearance of amide bands II due to the stretching of the C–N bond, for Val at 1590 cm−1 and 1620 cm−1 for Irbe. Thus, regarding the structure of Val, it can be seen that the –CO–N– fragment is not part of the imidazole cycle, but is "a free amide" and therfore the corresponding peak is located in the "true region of Amide band II" while in the case of the Irbe sample, this amide belongs to the imidazole ring, which makes it "less free," thus preventing the stretching of the C-N bond, and favoring the stretching of C=O, resulting in a shift of this peak to a higher wavenumber. Although due to the presence of the tetrazole ring, the νN=N should appear for all analyzed substances, it can only be seen in the case of Irbe and Los samples, this vibration being „covered” by the positioning of the Amide band II of the Val sample (as described earlier). Finally, for the Val and Los samples, due to the presence of the OH group, the characteristic νC-OH peak can be observed at 1000 cm−1.
Irbesartan exists as a mixture of tautomers A and B in the liquid state, which differ only in the mode of protonation of the tetrazole ring [18], which can be isolated in the solid state. Also, polymorphic forms can have different patterns in their vibrational spectra. Protonation of the tetrazole fragment leads to the delocalization of the electron density generating a new double bond between the nitrogen atoms, which directly leads to the appearance of absorption bands with an average intensity in the range of 1018–1237 cm−1. A characteristic band that allows distinguishing between the two forms can be the NNN bending, which in the case of the analyzed sample appears at the value of 1006 cm−1, and only in form B. Thus it can be said, that in the case of our sample, Irbe is in tautomeric form B.
In the FTIR spectrum of Los, the major peaks of the functional group were present according to the literature. The peak at 3180 cm−1 indicates the O–H stretching vibration, the C=C bond was assigned to the peak at 1496 cm−1, whereas the peak at 1188 cm-1 indicates the C-N bond [19, 20]. To be able to argue which polymorphic form is present in the Los sample, the peaks present in the range 1800–600 cm−1 were carefully analyzed. The differences between the two polymorphic forms are very difficult to highlight only based on FTIR data [21]. Considering the FTIR spectrum of the analyzed substance (Fig. 2) we can say that the sample contains a mixture of the two polymorphic forms because, as is known, in this range, the different molecular packing of the two polymorphic structures leads to the presence of different bands in the spectrum IR. Thus, according to the literature, in the case of the polymorphic form I, two bands are present at 764 cm−1 and 713 cm−1, while in the case of the polymorphic form II, due to the different arrangement, Van der Waals bonds between the molecules can no longer form and thus only one band will be visible around 754 cm−1. In the case of the imidazole ring, three absorption bands are observed in the range 850–970 cm−1 Form I and only one band at 940 cm−1 in Form II. In the aliphatic region, a peak around 1357 cm−1 is present in the case of Form II and is attributed to the symmetric C-H bending vibration in the methyl group of the n-butyl chain on the imidazole ring. The IR data suggest that the differences in absorption patterns between the two polymorphs are due to differences in intermolecular interaction in the two crystal forms. As can be seen in the FTIR spectrum of the Los sample, all the bands of both form I and of form II can be found, therefore we can say that the Los sample is a mixture of the two polymorphic forms. To validate the presence of one or another polymorphic form in larger quantities, we will compare the data with those obtained in the case of thermal analysis.
Analyzing the FTIR spectrum of valsartan and comparing it with data from the literature [22,23,24] it can be seen that the active substance is present in crystalline form by the presence of bands in the range 900–600 cm−1 and bands in the range 1732–1604 cm−1. Val in its crystalline form shows two carbonyl absorption bands, one at 1732.1 cm−1 and the other at 1603.8 cm−1 attributed to the stretching vibrations of the carboxylic carbonyl and of the amide carbonyl.
FTIR-UATR analysis of binary mixtures
In order to highlight the excipients that can be used in the tablet formulations, we also resorted to the study of binary mixtures obtained by mechanically mixing the active substance (Los, Irbe or Val) with each excipient separately in mass ratios of 1:1. Within the binary mixtures, the contribution of the active substance was monitored.
In Fig. 3, the FTIR spectra of the binary mixtures are presented comparatively. The characteristic bands are highlighted for easier validation of compatible chews. In the case of the FTIR study carried out on the binary mixtures with Irbe, it is observed that all excipients are suitable for pharmaceutical formulations in tablet form. All the bands characteristic of the active substance are present in the mixtures (Figs. 4, 5).
The FTIR analysis of the binary mixtures with Los indicates interactions between Los and Lactose, respectively Los and LactSBK when the C-H bands in the 2900–3000 cm−1 range are not visible and neither are those related to the N = N vibration. Therefore, we can support the fact that lactate-type excipients are not indicated in pharmaceutical formulations with Los. In the other binary mixtures, the characteristic bands of Los are visible. According to the literature, lactate as an excipient can interact with active substances containing amino groups [24].
According to the data obtained from the FTIR analysis for the combinations of Val and excipients, no changes were observed in the characteristic peaks of the active substance. One thing should be mentioned, namely, in the case of the MgSt and MgSTSZB combinations, an increase in the intensity of the peaks corresponding to the stretching vibration of C–H bonds is observed. This phenomenon is due to the stearate which, through its aliphatic chain, enriches the system with C-H bonds.
Thermogravimetric analysis
Thermal analysis of active substances
Thermogravimetric data were obtained in temperature range 25–400 °C, dynamic air atmosphere. Firstly, was study thermal behavior of active substance (Irbe, Los and Val) and then the binary mixture sartan-excipients. Same methodology was used for DSC study. Figure 6 presents data obtained for active substances.
The active substances were also analyzed in their pure form in order to have a source of comparison for the thermal behavior of the binary mixtures. The selected temperature range is sufficient to evaluate the thermal stability of all components and to obtain a clear picture of the processes that can occur in the air atmosphere even under extreme conditions. The stages of decomposition, the mass losses and the thermal effects that accompany these stages of decomposition, as well as the existence of their different polymorphic forms, were followed.
The thermal analysis of Irbe in the investigated interval shows three stages of decomposition, the last one is not completed until 400℃. Stability of the sample is observed up to 225℃. The only process that occurs up to 225℃ is the melting of the active substance, which has a maximum at 187℃, which is in close agreement with the literature data. According to literature data [25, 26], it can be argued that Irbe is present in the polymorphic form B, which has a higher melting point than the polymorphic form A. According to the literature, form A changes to form B at temperature. It is also possible to substantiate from the data obtained the fact that the stages of decomposition are complex and the degradation of the structure takes place in several stages that cannot be separated on the TG and DTG curves. In the studied temperature range, two processes accompanied by mass loss are observed, the first with a loss of 13.41% and the second with a loss of 33.63% with maxima on the DTG curve at 245℃, 345 and 397℃. Melting can be observed on the DSC curve and the existence of two further exothermic processes, the last of which is not completed in the temperature range investigated.
The thermoanalytical curves obtained for Los in the range of 25–400℃ highlight a single decomposition stage. The process starts at 275℃ and is accompanied by a mass loss of about 25% of the mass of the sample, which can be attributed to the loss of C5H8NCl [25]. As is known in the literature, two polymorphs have been found for the active substance Los – Form I and Form II. When the Form I material is heated, it transforms into Form II, followed by a melting process, and decomposes immediately after melting [27]. During the DSC analysis, it was highlighted that Los is present in the polymorphic form II, which was also supported by the FTIR study.
Los shows a much higher thermal stability than Irbe and Val, although the three active substances have similar structures. The thermal stability is due to the presence of stable cyclic structures and conjugation within the structure.
The thermogravimetric curves of Val indicate a lower stability of this substance compared to the two previously presented, a stability that is due to the slightly different structure compared to the other two. The decomposition starts at 157℃ and shows three processes on the TG and DTG curves, which are accompanied by mass loss. On the DSC curve, a first endothermic process is observed at 107℃ in close agreement with data from the literature, followed by two further endothermic processes, the last of which is not completed in the studied temperature range. Together with the FTIR spectroscopy and DSC thermal analysis data, we argue that the studied active substance is present in crystalline form [27].
The first phase of mass loss with a very low mass highlighted on the TG curve in the range of 35–75℃ can be attributed to the dehydration of the sample. Another process accompanied by mass loss occurs in the range of 157–285℃ with a mass loss of 19% and leads to decomposition and can be attributed to the loss of C5H8O. The last process, which is not completed until 400℃, starts at 285℃ and has a consistent mass loss, over 50% of the mass of the sample. The process can be attributed to the loss of a large part of the structure of the active substance, namely C12H15N3O2.
Thermal analysis of binary mixtures
In the case of binary mixtures, the presence of decomposition of the active substance was monitored. The absence of these processes or the change in the parameters of these processes may indicate possible interactions with excipients.
The analysis of the data obtained from the thermogravimetric study of binary mixtures with Irbe (see Fig. 7 and Table 1) shows that there is a characteristic mass loss stage of the active substance in all binary mixtures. On the DTG curves, the processes associated with the decomposition of the active substance can be observed in all cases. In some binary mixtures, the decomposition of the active substance takes place in the same temperature range as the decomposition of the excipient. On the DSC curves obtained in the case of binary mixtures, the process of Irbe decomposition can be observed with the maximum in the range of 188–192℃, lower than in the case of the pure active substance, which is however argued with the fact that we have mixtures. These conclusions are in close correlation with the mass losses associated with the two components.
The thermal analysis of the binary mixtures with Los (Fig. 8 and Table 2) highlights two categories of excipients, namely excipients that can be used in pharmaceutical formulations from the point of view of thermal behavior, namely talc, MgSt, PVP, HEC, SiO2, Mannitol, MgSTZB and Starch, where the decomposition process of the active substance can be observed at slightly shifted temperatures on the DTG and DSC curves compared to the values recorded in the case of the active substance. The second category of excipients is represented by Lactose and LactSLBK, for which we cannot observe any decomposition process of the active substance. From the thermogravimetric results, we can conclude that these excipients are not suitable for pharmaceutical formulations containing Los as active substance. For each excipient of the second category, interactions can be suspected to occur. Lactose is a reducing disaccharide, so a aldehyde-amine addition take place and leads to the formation of a Schiff base, which further cyclizes to form a glycosamine [28,29,30] (Fig. 9).
The thermal analysis carried out in the case of the binary mixtures with Val (Fig. 9) made it clear that the decomposition process of the active substance in two decomposition stages is visible in all samples except the binary mixture with HEC. In the case of the binary mixture with HEC, the decomposition is continuous, so that the two decomposition processes of the active substance cannot be separated on the TG and DTG curves, but the endothermic process is visible on the DSC curve related to the melting of the active substance, which indicates that this excipient also does not exhibit any thermally induced interactions with the active substance. Regarding the maxima measured on the DTG curves related to the decomposition of the active substance, it can be observed that in the case of the binary mixtures with mannitol, MgSTSZB, LactSLBK and Lactose, the maxima are at the same value as in the case of the pure active substance, which leads us to conclude that Val does not interact with these excipients. For the other excipients, the decomposition process takes place with a maximum at higher temperatures. To illustrate the results, we follow the melting process of the active substance using the DSC curves of the mixtures. It can be observed very clearly that in all mixtures the endothermic processes related to the melting process of Val are at the same or slightly lower value, namely between 102 and 104℃, which according to the literature suggests that Val is present in a mixture. [31, 32]
So, we can say that based on the results of the thermal study, all excipients analyzed are compatible with the active substance Val (Table 3).
Conclusions
Our study evaluated the potential interactions between the three drugs of the sartan class (Irbesartan, Losartan and Valsartan) and various pharmaceutical excipients to obtain pharmaceutical tablet formulations. These sartans were chosen because they have similar structures and normally we could suspect a similar behavior. In the specialized literature, some compatibility studies of some drugs from the sartan class but with other excipients are presented.
Thermal analyses provided information on the thermal stability of active substances, polymorphic forms and decomposition binary mixtures with excipients, which provide information that can be used for quality control.
The present study validates by using thermoanalytical and FTIR spectroscopic techniques the use of excipients: Starch, HEC, MgSt, Mannitol, SiO2, Talc, Lactose and LactSLBK and PVP in tablet formulations with Irbe and Valsartan. In the case of Losartan, the same methods led to the conclusion that lactose and LactSLBK would present possible interactions with the active substance. In DSC studies, the changes observed in the curves suggested a possible interaction with the active substance through the lack of thermal effects accompanying the decomposition of the active substance. The FTIR study highlighted the absence of bands characteristic of the active substance in the same situation. These results provided a better understanding of the solid–solid interaction that can occur in studies of multicomponent solid pharmaceuticals that contribute to rational drug design.
References
Timmermans PB. Angiotensin II receptor antagonists: an emerging new class of cardiovascular therapeutics. Hypertens Res. 1999;22:147–53. https://doi.org/10.1291/hypres.22.147.
Mirabito Colafella KM, Bovée DM, Danser AHJ. The renin-angiotensin-aldosterone system and its therapeutic targets. Exp Eye Res. 2019;186: 107680. https://doi.org/10.1016/j.exer.2019.05.020.
Muszalska I, Sobczak A, Dothán A, Jelińska A. Analysis of sartans: a review. J Pharm Sci. 2014;103:2–28. https://doi.org/10.1002/jps.23760.
Mizuno K, Niimura S, Tani M, Saito I, Sanada H, Takahashi M, Okazaki K, Yamaguchi M, Fukuchi S. Hypotensive activity of TCV-116, a newly developed angiotensin II receptor antagonist, in spontaneously hypertensive rats. Life Sci. 1992;51(20):183–7. https://doi.org/10.1016/0024-3205(92)90627-2.
Lawson EC, Shook BC, Lanter JC. Tetrazole-based angiotensin II type 1 (AT1) antagonists for the treatment of heart failure and congestive hypertension. In: Dinges J, Lamberth C, editors. Bioactive Heterocyclic compound classes: pharmaceuticals. Bioorg. Wiley-VCH; 2012. pp. 153–167.
Furukawa Y, Shoji K, Kohei N. Hypotensive imidazole-5-acetic acid derivatives. Takeda Chemical Industries, 1982. US Patent US4355040A.
Swann S, Bigi S. Angiotensin II receptor antagonists with carboxylic functionalities in cardiovascular disease. In: Lamberth C, Dinges J, editors. Bioactive carboxylic compound classes: pharmaceuticals and agrochemicals. Wiley-VCH; 2016. p. 87–101.
Borghi C, Cicero AF. The role of Irbesartan in the treatment of patients with hypertension: a comprehensive and practical review. High Blood Press Cardiovasc Prev. 2012;19:19–31. https://doi.org/10.2165/11632100-000000000-00000.
Al-Majed AR, Assiri E, Khalil NY, Abdel-Aziz HA. Losartan: comprehensive profile. Profiles Drug Subst Excip Relat Methodol. 2015;40:159–94. https://doi.org/10.1016/bs.podrm.2015.02.003.
Martin J, Krum H. Role of valsartan and other angiotensin receptor blocking agents in the management of cardiovascular disease. Pharmacol Res. 2002;46:203–12. https://doi.org/10.1016/s1043-6618(02)00092-0.
Chaudhari SP, Patil PS. Pharmaceutical excipients: a review. Int j adv pharm biol chem. 2012;1:21–34.
Elder DP, Kuentz M, Holm R. Pharmaceutical excipients—quality, regulatory and biopharmaceutical considerations. Eur J Pharm Sci. 2016;87:88–99.
Rowe RC, Sheskey PJ, Quinn ME. Handbook of pharmaceutical excipients. 6th ed. Pharmaceutical Press and American Pharmacists Association; 2009.
Buda V, Baul B, Andor M, Man DE, Ledeţi A, Vlase G, Vlase T, Danciu C, Matusz P, Peter F, Ledeţi I. Solid state stability and kinetics of degradation for candesartan—Pure compound and pharmaceutical formulation. Pharmaceutics. 2020;12(2):86:1–17; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7076474
Franca CA, Etcheverry SB, Pis Diez R, Williams PAM. Irbesartan: FTIR and Raman spectra. Density functional study on vibrational and NMR spectra. J Raman Spectrosc. 2009;40:1296–300. https://doi.org/10.1002/jrs.2282.
Billes F, Endredi H, Keresztury G. Vibrational spectroscopy of triazole and tetrazole. J Mol Struc-Theochem. 2000;530:183–200.
Peica N, Lehene C, Leopold N, Schlucker S, Kiefer W. Monosodium glutamate in its anhydrous and monohydrate form: Differentiation by Raman spectroscopies and density functional calculations. Spectrochim Acta A Mol Biomol Spectrosc. 2007;66:604–15. https://doi.org/10.1016/j.saa.2006.03.037.
Franca CA, Etcheverry SB, Pis Diez R, Williams PAM. Irbesartan: FTIR and Raman spectra density functional study on vibrational and NMR spectra. J Raman Spectrosc. 2009;40:1296–300. https://doi.org/10.1002/jrs.2282.
Pahuja S, Aggarwal S, Sarup P. Formulation and characterization of Losartan loaded Chitosan microspheres: effect of crosslinking agents. Drug Res. 2021;71:204–12. https://doi.org/10.1055/a-1324-2466.
Bhairam M, Prasad J, Verma K, Jain P, Gidwani B. Formulation of transdermal patch of Losartan Potassium & Glipizide for the treatment of hypertension & diabetes. Mater Today:Proc. 2023;83:59–68. https://doi.org/10.1016/j.matpr.2023.01.147.
Raghavan K, Dwivedi A, Campbell GC Jr, Johnston E, Levorse D, McCauley J, Hussain M. A spectroscopic investigation of losartan polymorphs. Pharm Res. 1993;10:900–4. https://doi.org/10.1023/a:1018973530443.
Usha Sri B, Indira Muzib I, Bhikshapathi D, Sravani R. Enhancement of solubility and oral bioavailability of poorly soluble drug valsartan by novel solid self emulsifying drug delivery system. Int J Drug Deliv. 2015;7:13–26.
Kale KB, Shinde MASA, Patil RH, Ottor DP. Exploring the interaction of Valsartan and Valsartan-Zn (II) complex with DNA by spectroscopic and in silico methods. Spectrochim Acta A Mol Biomol Spectrosc. 2022;264: 120329. https://doi.org/10.1016/j.saa.2021.120329.
Júlio TA, Zâmara IF, Garcia JS, Trevisan MG. Compatibility and stability of valsartan in a solid pharmaceutical formulation. Braz J Pharm Sci. 2013;49:645–51.
Wardhana YW, Riskasari R, Alatas F. Phase Transitions among of Valsartan polymorphs due to grinding and humidity variations. Indo J Pharm. 2021;3:82–7.
Khattab WM, Gad S, El-Sayed MM, Ghorab MM. Simple controlled release delivery system for an anti-hypertensive drug via buccal route. Br J Pharm Res. 2014;4:1174–95.
Zong YJ, Wu Cheng J, Wang XJ. The thermal decomposition mechanism of irbesartan. J Anal Appl Pyrolysis. 2018;134:93–101. https://doi.org/10.1016/j.jaap.2018.05.014.
Bharate SS, Bharate SB, Bajaj AN. Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: a comprehensive review. J Excipients and Food Chem. 2010;1(3):4–26.
Flemming A. Compaction of lactose drug mixtures: quantification of the extent of incompatibility by FT-Raman spectroscopy. Eur J Pharm Biopharm. 2008;68(3):802–10. https://doi.org/10.1016/j.ejpb.2007.07.012.
Bengescu C, Ledeți A, Olariu T, et al. Instrumental investigations of promestriene: first report regarding the solid-state characterization and compatibility with pharmaceutical excipients. J Therm Anal Calorim. 2023;148:4641–9. https://doi.org/10.1007/s10973-023-11942-7.
Budiul MM, Marioane CA, Bradu IA, et al. FTIR and thermal studies of medicated jellies with topiramate. J Therm Anal Calorim. 2023;148:4589–600. https://doi.org/10.1007/s10973-022-11882-8.
Mateescu M, Vlase G, Budiul MM, et al. Preliminary study for preparation and characterization of medicated jelly based on Ibuprofen or Ambroxol. J Therm Anal Calorim. 2023;148:4601–14. https://doi.org/10.1007/s10973-023-12052-0.
Author information
Authors and Affiliations
Contributions
Conceptualization, B-D.C, I-A.B, D.O., and G.V; data curation, M-M.B., T.V., I-A.B., A.P. and G.V.; formal analysis, G.V., D.O., I-A.B. and T.V.; investigation, B-D.C., D.O., M.M.B., A.P. and T.V.; methodology, G.V., I-A.B., D.O. and T.V.; supervision G.V. and T.V.; validation, T.V. and G.V.; visualization, M.M.B., I-A.M., D.O. and T.V.; writing-original draft, D.O., G.V., B-D.C., and M.M.B. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cernușcă, BD., Bradu, IA., Pahomi, A. et al. Study of thermally induced interactions between active substances from the sartans class and various excipients. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13477-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13477-x