Abstract
Two approaches to the synthesis of hydrogels based on polyacrylamide (pAAm) with copolymers were compared in the paper—traditional chemical cross-linking and physical cross-linking with montmorillonite (MMT). The main aim of the work was to find an adequate replacement of the chemical toxic cross-linking agent MBAAm (N,N'-methylene-bis-acrylamide) by using non-toxic—natural clay MMT for synthesis of pAAm gels, which are planned to be used as soil conditioners. A series of hydrogels based on acrylic monomers (acrylamide (AAm), acrylonitrile (AN), acrylic acid (AA)) physically cross–linked by MMT and chemically cross-linked were synthesized. For the synthesized gels, the influence of the synthesis method on the formation of the structure and the mechanism of thermal destruction in the presence of air was analyzed using a set of physicochemical methods: FTIR, XRD, SEM, DSC and TG/DTG. According to FTIR and XRD data, pAAm-MMT and pAAm-AN-MMT samples formed an intercalated/exfoliated structure, whereas pAAm-AA-MMT had an intercalated structure. The endothermic reaction of decomposition of xerogels based on acrylic polymers with and without MMT was observed using DSC and derivative thermogravimetry analyses, coupled with measurement of FTIR spectra of volatile products of thermolysis. All studied composites were relatively thermoresistant, which had three distinct regions of phase transitions and their thermal decomposition occurred at a temperature range 310–465 °C.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acrylic polymer hydrogels are cross-linked (co)polymers capable of absorbing and retaining large volumes of liquids. Due to their high sorption abilities, hydrogels have found application in various areas of human life, such as agriculture [1], ecology [2, 3], pharmacology and medicine [4,5,6,7,8]. In medicine, hydrogels are mostly used in soft tissue engineering of skin: as applied plasters for healing wounds and burns, for targeted delivery of drugs to the affected area [8]. Hydrogel conditioners are one of the most modern additives to agricultural soils and valuable materials applied during the remediation of the water-soil environment. They are used to increase water retention as well as prolong the release of mineral fertilizers and pesticides [1, 9,10,11,12].
One of the most well-known and widely used methods for the synthesis of acrylic polymer hydrogels is radical polymerization [13]. The majority of hydrogels described in the literature so far are chemically cross-linked ones. In their structure, linear polymer chains are bonded by covalent bonds through bifunctional cross-linking agents [14]. Various substances containing multiple bonds are used as covalent cross-linkers, such as N,N'-methylene-bis-acrylamide (MBAA) and divinylbenzene. [15, 16]. The amount of the cross-linking agent affects the sorption characteristics and physicomechanical properties of the resulting polymers due to changes in the cross-linking density [16, 17]. MBAA cross-linked hydrogels always have some defects in network density caused by synthesis conditions, quality, the reactivity of components, network formation mechanism, etc. [16,17,18].
Another type, physically cross-linked hydrogels, formed mainly due to weak non-covalent interactions, such as van der Waals forces or hydrogen bonds, are described much less frequently [19,20,21]. One of the latest trends is using two-dimensional (2D) clay minerals for hydrogel cross-linking [21, 22]. The clay minerals of smectite group, e.g., natural montmorillonite (MMT) [23,24,25] and synthetic LaponiteRD [26,27,28], are the most widely used to prepare nanocomposite hydrogels mainly due to their specific layered structure, significant swelling in water, and cation-exchange capacity [29, 30]. Clays can form hydrogen and ionic bonds with hydrophilic polymers due to their internal chemical composition, e.g., the presence of silanol groups and negative charge on the surface [31]. Due to its distribution in nature, low cost, high surface area, hydrophilicity, and environmental friendliness, not toxicity for plants and animals, MMT is the most widely used clay [32]. A wide application of MMT in the preparation of hydrogel composites is usually a result of its high ability to stratify into individual plates with a thickness of about 1 nm and a diameter of 20–250 nm under certain conditions [33]. As an additive, clays can increase the stability of nanocomposite systems and improve their physicomechanical characteristics, such as strength, elasticity, retention of the sample shape in a swollen state, and increase the sorption capacity. The preparation of hydrogel materials with special properties and acceptable performance characteristics (in particular, a combination of high sorption capacity and strength) was carried out with various inorganic fillers and cross-linking agents [23,24,25,26,27,28,29,30,31, 33,34,35,36,37,38,39,40]. Significant practical results in this area have been obtained using MMT; however, the still insufficient study of the effect of "composition-structure–property" of MMT-containing polymers and composites based on them hinders the possibility of manufacturing such products from a purely technological point of view. Most of the studies focused on the swelling, sorption, and release properties of MMT-acrylic polymer composite hydrogels [23, 24, 35, 38, 41]. The incorporation of small amounts of hydrophilic clay nanoparticles as additives into a polymer hydrogel can contribute to increased water absorption capacity, depending on the dispersion level, but it can also decline swelling due to its cross-linking properties [22]. Only a few papers compared the properties of chemically and clay-cross-linked hydrogels regarding their mechanical properties [41, 42]. The MMT-cross-linked pAAm hydrogel showed extraordinary stretchability: It could be stretched up to 11 800% without fracturing, in contrast to the MBAA-gel that fractured at 68% strain [41]. Also, these composites fully recovered at room temperature after stretching and self-healed through a drying-reswelling procedure. The elongation at break of LaponiteXLS-cross-linked pAAm was also much higher (by 70 times) than that of chemically cross-linked gel [42]. Thermal properties of such composite materials, in particular the influence of clays on the thermal destruction of composite hydrogels, have received insufficient attention in the literature, although the introduction of clay minerals into gel composites may be promising by improving their thermal stability and stability to other environmental factors. It was reported that traditional MBAA-cross-linked polyacrylamide (pAAm), in addition to structure heterogeneity, demonstrated insufficient thermal stability and a tendency to syneresis at high temperatures due to the destruction of polymer chains, the breaking of cross-linking agent molecules, and hydrolysis [43]. In some papers [22, 44,45,46], it was emphasized that clay introduction into the hydrogel matrix improved materials' thermal resistance compared to the unfilled hydrogels. The thermogravimetric measurements showed that adding MMT and bentonite to pAAm increased the onset temperature of degradation by 56 °C and 58 °C, respectively [46]. Similar increase (by 30 °C) was reported for pAAm/alginate-montmorillonite composite [46]. Increasing clay content from 25 to 45.5% in LaponiteXLS-cross-linked pAAm hydrogel increased the thermal stability of the hydrogels [26]. Poly (acrylic acid-co-N-isopropylacrylamide)-LaponiteXLS composite exhibited higher thermal stability at the increasing of clay and the decreasing of acrylic acid (AA) content [47].
The above determines the relevance of the problem of creating new physically cross-linked hydrogel nanocomposites to an increase in deformation-strength characteristics, an increase in the modulus of elasticity, sorption properties and improved thermal stability. This work aimed to compare structure and thermal properties of MMT and MBAA cross-linked pAAm, pAAm-AA and pAAm-AN hydrogels. The effectiveness of regulating the properties of filled polymer composite materials based on acrylic hydrogels containing MMT particles by changing various factors, namely the use of different monomers and the introduction of the optimal amount of mineral filler was studied.
In the near future, physically cross-linked hydrogels should be considered as artificial soil conditioners for agricultural purposes with a complex effect. They can combine optimal soil moisturizing, enhanced water holding properties, as well as sorption properties regarding heavy metals and the ability to prolonged release of nutrients (mainly micro- and macroelements). Since the use of traditional hydrogels in medicine and agrotechnology is limited due to the toxicity of the corresponding monomers and cross-linking agents, it seems unavoidable to move to the use of biosafe natural polymers and hydrogels on their base. This article is devoted to the search for ways of physical cross-linking using the natural clay mineral—MMT, which allows to avoid the toxic effects of traditional chemical cross-linking agents such as MBAAm. Further efforts are planned to be directed to the synthesis of completely bio-safe hydrogels, e.g., created based on polysaccharides. So far, the most popular hydrogels used in agriculture have been those synthesized from acrylamide and acrylate derivatives. The selection of cross-linking agents is crucial for the hydrogel biocompatibility. Those that are cross-linked by hazardous MBAAm require specific washing procedure before application. Replacing this substance with natural clay minerals will reduce environmental risks and the costs of material production.
Materials and methods
Materials
The following starting monomers were used for the synthesis of hydrogels: Acrylamide (AAm, Merck, 99%), Acrylic acid (AA, Merck, 99%) and Acrylonitrile (AN, Sigma-Aldrich, 99%). Acrylic acid and Acrylonitrile were distilled with the addition of 1 mL of concentrated sulfuric acid for removing of polymerization inhibitor (hydroquinone).
Potassium persulfate K2S2O8 (PPS; Sigma-Aldrich, 98%), N,N,N′,N′-tetramethylethylenediamine (TEMED; Sigma-Aldrich, 99%), N,N′-methylenebisacrylamide (MBAAm, Merck, 98%) were used in chemical cross-linking process. Montmorillonite K10 powder (Na-MMT, Sigma-Aldrich, SBET ~ 250 m−2g−1) was used as received without further purification for physical cross-linking. Double-distilled water was used as a solvent in all experiments.
Synthesis of chemically cross-linked hydrogels
Hydrogels based on AAm and its copolymers with AN and AA were synthesized by radical polymerization of monomer aqueous solutions at 40 °C and simultaneously flowing covalently cross-linking using bifunctional monomer MBAAm (Tables 1 and S1, Fig. S1, S2 see in ESM file). The content of the cross-linking agent in the gel-forming composition was 0.64 mass% by mass and was chosen empirically, taking into account some properties of the formed hydrogels requested for their using, namely their ability to retain their original shape during hydration and the degree of swelling.
For initiation of the polymerization, a redox system based on PPS and TEMED was used. The hydrogel synthesis was performed as follows. Argon was bubbled through a reaction mixture (aqueous solutions of monomers and cross-linking agent) before adding the redox system. After the addition of the redox system, the composition was mixed and transferred to a mold consisting of two parallel glass plates separated by 1-mm-thick spacers. Approximately four hours later, the polymerized hydrogels were removed from molds and washed extensively in distilled water at room temperature to remove unreacted residues. Water was changed 1–3 times a day, and the washing process was controlled using a UV–Vis spectrometer (SPECORD M40, Carl Zeiss). Gel disks were cut from swollen hydrogel films using a hole puncher (d = 10 mm) and dried to a constant mass at 25 °C.
Synthesis of physically cross-linked hydrogels
Synthesis of physically cross-linked hydrogels was performed by a similar method using montmorillonite (MMT) instead of MBAAm as a cross-linker (Fig. S3, S4). The concentration of MMT in the case of physical cross-linking of acrylic monomers was higher than in the case of chemical cross-linker (0.64 mass%) and depended on copolymers structure: for reaching optimal structural strength the MMT concentration amounted to 5.9 mass% for AAm, 4.3 mass% AAm-AN, and 9.9 mass% for AAm-AA hydrogels.
Fourier transform infrared (FTIR) spectroscopy
FTIR spectra of powdered samples over the 4000–400 cm−1 range were recorded using a ThermoNicolet iS10 FTIR spectrometer with the ATR method.
Scanning electron microscopy (SEM)
Morphological analysis in the microarray of the studied materials was carried out on a Quanta 250 FEG scanning electron microscope from FEI equipped with an EDS attachment from EDAX. The experiments were conducted on samples sprayed with a conductive carbon layer at an accelerating voltage of 15 keV.
X-ray diffraction (XRD)
Phase compositions of the solids were characterized by X-ray diffraction on a Panalytical XPert Pro MPD. A copper lamp (CuKα = 1. 54,178 Å) was used as the emission source. The test was conducted over an angular range of 5–65° 2Θ with a step equal to 0.02° 2θ lasting 5 s. X'Pert Highscore software was used to process the diffraction data.
Thermal analysis
Thermal analysis was carried out using a STA 449 Jupiter F1 (Netzsch, Germany) apparatus, sample mass ~ 16 mg placed into a corundum crucible, air flow of 50 mL min−1, a heating rate of 10 °C min−1, temperature range of 30–950 °C, and S TG-DSC sensor thermocouple type. Empty corundum crucible was used as a reference. The gaseous products emitted during decomposition of the materials were analyzed by using a FTIR Brucker (Germany) spectrometer. The FTIR spectra were recorded in the range of 4000–600 cm−1 with 16 scans per spectrum at a resolution of 4 cm−1.
Results and discussion
FTIR spectra of chemically and physically cross-linked pAAm-based gels
Since N,N'-methylene-bis-acrylamide was used for the synthesis of chemically cross-linked hydrogels based on acrylamide and copolymers with acrylonitrile and acrylic acid, the structure of the spatially cross-linked gel includes functional groups characteristic of each of the monomers and MBAAm, which forms cross-bridge bonds between macromolecular chains. The FTIR spectra of pAAm-based gel samples (Fig. 1, S5–S7) are characterized by the presence of specific absorption bands, which are attributed to acrylamide fragments of polymer macrochains (Fig. S1a, scheme). The pAAm-AA co-polymer additionally contains a carboxyl group in its composition (its content is proportional to the content of AA, Fig. S1b, Scheme), and pAAm-AN is characterized by an additional nitrile (cyano) group, –C≡N, in the structure (Fig. S1c, Scheme).
In the FTIR spectra of all chemically cross-linked gels based on pAAm, intense stripes in the range of 3000–2800 cm−1 are observed, regardless of the nature of copolymers (AN or AA) (Fig. 1a). The valence stretching of C–H of the studied systems is characterized by two peaks, 2898 and 2966 cm−1 corresponding to symmetric and antisymmetric oscillations in CH2 groups of polymer alkyl chains [48]. It has been noted that the shape and position of the C–H strip is not affected by the presence of MMT in the structure of hydrogels (in the case of physical stitching of hydrogels). This indicates that acrylamide does not violate the order of packaging and/or conformational dynamics of alkyl chains. In the area of "fingerprints" the doublet at 1449 and 1412 cm−1 is observed for chemically and physically cross-linked gels. These bands were assigned to bending vibrations of the C–H group (δC-H in CH2) and the C–H overlapping area (δC–H in CH and τC–H, ωC–H in CH2) [48, 49].
The functional group of amides, included in the synthesized compositional group of gels, combines the features of amines and ketones, since it has both N–H bond and C = O. Therefore, amides show a very strong, slightly wider strip in the long-wave region of the spectrum, in the range of 3100–3500 cm−1 of the bond N–H stretching. NH2 stretching vibrations appeared at 3194, 3336 cm−1 (duplicate characteristics of primary amines) for pAAm-based gels chemically cross-linked and physically cross-linked by MMT [49]. The shoulder of about 3422 cm−1 can be attributed to the fluctuations in the OH bond in the carboxyl group of acrylic acid, as a component of pAAm-AA gel. Intensive maximum at 1649 cm−1 corresponds to a fragment of Amide I and is associated with stretch vibrations ν (C = O), which is covered with the maximum of deformation vibrations (δN-H) (NH2) = 1608 cm−1 (Amide II) with the formation of an extended doublet [50].
For acrylamide-acrylonitrile copolymer peak at 2241 cm−1 refers to the functional group of nitrile (cyno) C≡N [51]. When MMT is used as a sewing agent, there is a slight shift of the peak (at 2245 cm−1), which can occur due to the interaction of cyano groups with MMT. Low intensity bands at 1103–1174 cm−1 are observed in IR spectra of chemically and physically cross-linked composite gels, which can be attributed to both twisting -NH2 and deformation (fan) vibrations of –CH2 groups [52]. Characteristic peaks of Na-MMT at 1088 cm−1 (Si–O stretching out of plane), 988 cm−1 (Si–O stretching in plane), 912 cm−1 (bending AlAlOH), 873 cm−1 (bending AlFeOH), 797 cm−1 (bending Si–O), 514 cm−1 (bending Si–O–Al), 3620 (stretching AlAl–OH) and 3425 cm−1 (H2O) and 1635 cm−1 (O–H bending, hydration) are observed in the spectrum of the initial MMT (Fig. S5a) [53,54,55].
In the case of cross-linking of monomers by a physical method using MMT as a cross-linking agent, embedding in the inter-packer space of MMT plates, intercalated monomers interact most likely through the formation of hydrogen and electrostatic bonds between the amide group or different polar (CONH2, COOH, CN) groups of monomers and oxygen atoms of the MMT crystal lattice. At the same time, the structure of MMT changes as a result of interaction with acrylic monomers. In general, with the introduction of MMT into the mixture during the synthesis of a polymer composite, there are possible options for the formation of intercalated, exfoliated and conventional (flocculated) structures (Fig. S3), which are characterized by different distances between the mesh layers of clay (MMT). In our case, the presence of an intercalation structure (Fig. 1b, S6–S7) is evidenced by the shift of the Si–O band (centered at 988 cm−1 for the pristine MMT) toward higher wave numbers for composites (1023–1030 cm−1) [56]. As it is known, a broad band of Si–O–group at 988 cm−1 in FTIR spectra of MMT is sensitive to intercalation [57]. A slight increase in the distance between the MMT layers is observed due to the intercalation of monomers in the interlayer space (Fig. S3), or the distances between the MMT layers can be greater due to the formation of exfoliated structure. Thus, the shift of these bands to 1023–1026 cm−1 for MMT-cross-linked hydrogels indicates an increase in interlayer distance and mixed intercalated/exfoliated structure formation. The absence of another absorption peak of Na-MMT in the FTIR spectra of the nanocomposites also indicates the exfoliation of the MMT plates in the pAAm-based polymers.
SEM
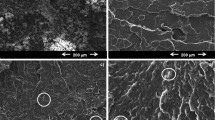
The surface morphologies of the pristine MMT and PAM-based MMT composites and also the distribution of the MMT layers in the polymer matrix were examined by SEM.
As it is shown in Fig. 2a, MMT exhibited an aggregated and sheet-like plate structure before the intercalation. From the SEM image (Fig. 2a) of the pristine MMT, a layered structure with individual scales 100–300 nm in size can be observed, which are overlapped together (stacked in aggregates). The known concept of layered clays arrangement indicate that clay minerals stick to each other at the points of contact, with forces sufficiently strong to construct a honeycomb structure [56]. In relation to such a structure, a model is used [56] which include (i) the domain (the structure formed by clay primary particles), (ii) the cluster (the structure formed by the domains gathering around silt and clay), and (iii) clumps. Generally, layer thickness in the layered silicates is in the order of 1 nm and a very high aspect ratio (e.g., 10–1000). Thus, the peculiarities of the structure of layered silicates provide high surface area interaction with polymer matrix at structure formation. construct a honeycomb structure [56].
From SEM images of pAAm-based MMT composites (Fig. 2b–d) it is noticeable that cross-linking monomers by MMT leads to formation of a regular uniform cluster (periodic) structure, in which elementary structural elements can be traced in the form of domains (scales) of MMT covered with the polymer layer. SEM images of chemically cross-linked gels (Fig. 2e, i) show a smooth non-porous amorphous surface of the polymer. The SEM images of pAAm-MMT and pAAm-AN-MMT (Fig. 2b, c), for which the MMT content is 5.9–5.7, respectively, show a cluster structure on the surface of the composite, which proved uniform binding of monomers by MMT and taking into account agreement with FTIR and XRD data corresponds to intercalate/exfoliate structure. In the case of the pAAm-AA-MMT composite, the ratio of the initial components with a higher MMT content (Table 1) was used to obtain the cross-linked structure, however, despite this, the composite surface is a solid polymer surface according to SEM (Fig. 2d).
This effect may be associated with a worse interaction between MMT and AA due to the same (negative) surface charge of MMT and AA in aqueous media, which leads to a worse distribution of MMT in the structure of the composite and reduced cross-linking abilities of MMT it in relation to AA monomers. In this case, the formation of a convection structure is also possible, and the structure of pAAm-AA-MMT composite corresponds to intercalate/convention one, which is in agreement with the XRD data given in the next subsection.
X-ray powder diffraction
A Fig. 3 shows the XRD patterns of MMT and MMT-containing hydrogels. XRD is one of the most important techniques to determine the structural geometry and texture of clays. The reflections relative to the planes [001] and [002] confirmed the presence of MMT as main phase. The XRD of the PAM-based gels cross-linked by MMT revealed a shift in the position of [001] planes, meaning an increase in the basal spacing of these planes. The characteristic peak of MMT appears at 2θ = 7.2 and corresponds to the clay interlayer distance d001 of 1.22 nm. In the case of pAAm-AA-MMT, the characteristic peak shifts to 2θ = 6.4, which correspond to a distance of 1.41 nm.
This result corresponds to intercalated or partially exfoliated structure formation with higher interlayer distance [58, 59]. In the case of pAAm-MMT and pAAm-AN-MMT samples, the mentioned peak disappears, corresponding to the exfoliation of MMT layers. From the XRD patterns in Fig. 3, it is also observed that the peaks from [002] planes of MMT, were not changed as a result of synthesis. Similarly, the exfoliated structure formation of clay-cross-linked nanobentonite/pAAm, nanoMMT/methacrylic acid, and laponite/pAAm hydrogel composites was confirmed due to the disappearance of clay basal peaks in diffractograms [34, 35, 60]. Zhou et al. synthesized pAAm-AA-MMT composites with different MMT-to-monomers ratios [23]. The gradual decrease until the disappearance of the MMT peak was observed with the increase in monomer content. This could be associated with interlayer distance increase and transition from intercalated to exfoliated structure depending on the clay content in the hydrogels.
Thermal analysis
The thermal characteristics (TG, DTG, and DSC) of pAAm, pAAm-AN, pAAm-AA and pAAm-MMT, pAAm-AN-MMT, pAAm-AA-MMT were studied upon heating of samples in air (Figs. 4, 5 and Table 2). Thermal decomposition of organic molecules is very complicated and occurs in a few main stages. As it can be seen from the DSC and TG/DTG (Figs. 4 and 5) curves, four main stages of thermal destruction are observed for all studied gels.
The stages comprising physicochemical transformation (dehydration, melting, changes in conformation of molecules, initial defragmentation etc.) occur at relatively low temperature. In the case of the analyzed samples, first slight mass losses (up to 9.7%, Table 2) to the temperature of about 210 °C (170 °C in case of pAAm-AA) are related to the endothermic removal of physically bound water (despite initial pre-drying at 50 °C), and other components that are poorly bonded to the surface, which suggests the presence of the weak absorption bands in FTIR spectra in the range of 3800–3500 cm−1 (Fig. S8).
Thermal transformations of similar chemically cross-linked gels based on acrylamide and acrylic acid without pre-removal of physically sorbed water were studied previously using temperature-programmed mass spectrometry (TPD-MS) in the temperature range from 30 to 800 °C [61]. The authors noted that the mass spectra of all studied hydrogels in the temperature range from room to approximately 250 °C are characterized by the presence of lines associated with the release of water, primarily m/z 18 (H2O+) and m/z 19 (oxonium ion H3O+), as well as the low-intensity signals with values of m/z 17 and 16 that at 120 °C there are also observed, which can be associated with the separation of NH +3 and NH +2 fragments, respectively. This mechanism was confirmed by FTIR spectra of the volatile components of the 1st stage of thermal destruction (Fig. 6A).
FTIR spectra of volatile products thermal destruction of pAAm-based hydrogels chemically cross-linked (1—pAAm, 3—pAAm-AN, 5—pAAm-AA) and physically cross-linked by MMT (2—pAAm-MMT, 4—pAAm-AN-MMT, 6—pAAm-AA-MMT) and schemes of reactions: (A) at 1nd stage (Tmax = 30–220 °C); and (B) at 2nd stage (Tmax = 220.2–269.3 °C) and 3rd stage (Tmax = 372.1–395.7 °C)
At higher temperatures, the next decomposition stages correspond to processes of partial defragmentation and organic decomposition. Thus, during the 2nd stage of thermal transformations of composites in the temperature range 220–320 °C, on the FTIR spectra of volatile products released at this stage, only a few main peaks are observed at 3330, 1625 cm−1, 966 and 930 cm−1 (Fig. 6B). These processes are associated with the separation of amino groups and the release of volatile ammonia compounds or primary amines. Thus, NH3 has a characteristic absorption band, or fingerprint, mainly consisting of two distinct absorption peaks at 965 and 930 cm−1, respectively. This fingerprint line can be clearly identified in the FTIR spectra of volatile products for all pAAm-based hydrogel samples (Figs. 6, 7b and S8).
Comparison of FTIR spectra for main products in evolved gases of pAAm samples (1—pAAm, 2—pAAm-AN, 3—pAAm-AA, 4—pAAm-MMT, 5—pAAm-AN-MMT, 6—pAAm -AA-MMT): (a) H2O (3730–7733 cm−1), (b) NH3 (927–930 cm−1), (c) CO (2108–2111 cm−1), (d) CO2 (2349–2359 cm−1), (e) imide (3401–3409 cm−1) and (f) nitrile (2243–2250 cm−1) compounds as well as (g) –CH3 (2959–2976 cm−1) –C = O (1739–1748 cm−1) groups
The N–H stretches of amines are in the region 3350–3000 cm−1. For primary amines (RNH2), there are two bands in this region, the asymmetrical N–H stretch and the symmetrical N–H stretch. The N–H bending vibration of primary amines is observed in the region 1650–1580 cm−1. Usually, secondary amines do not show a band in this region and tertiary amines never show a band in this region. Another band attributed to amines is observed in the region 910–665 cm−1. This strong, broad band is due to N–H wagging out-of-plane and observed only for primary and secondary amines. That is, the process of thermal destruction of all synthesized pAAm-based hydrogels starts from defragmentation and destruction of functional amino groups in the structure of hydrogels without destruction of the polymer backbone. These processes are endothermic reactions. In the case of the pAAm sample, the process occurs in three stages with the mass loss of 15.09%, as evidenced by the peaks on the DTG curves with a minimum temperature of 233, 251 and 305 °C (Fig. 4, black line). In the case of the sample synthesized with the addition of acrylonitrile, a significant increase in the amount of separated gaseous products (by about 5% compared to pAAm) and a similar three-stage decomposition were observed. Analysis of FTIR spectra of gaseous decomposition products at particular temperatures (Figs. 7 and S8) showed that NH3 was the main product in the whole range. In contrast to pAAm and pAAm-AN, the defragmentation process of the pAAm-AA sample takes place in one step (Fig. 4, red line) at a much lower temperature (Tmax = 220 °C) and the mass loss is similar to pAAm (Table 2). These changes are related to the decomposition of the functional groups of the acrylic acid used in the synthesis, as a result of which water is emitted Fig. 7a. In the case of physically cross-linked hydrogels (Fig. 5), the mass losses are much smaller due to the lower amount of acrylic compounds used. It appears that the presence of MMT does not affect the mechanism of the process (the major decomposition product was NH3) but it causes DTG peaks to shift toward higher temperatures, and for pAAm-AA-MMT also an extra peak on DTG curve is visible.
The 3rd stage of thermal destruction (peak at 320–445 °C) on the DSC and TG/DTG curves corresponds to the complex organic decomposition of polymer molecules that make up the hydrogel structure. In general, Tmax (temperature of peaks on DTG curves, Table 2) of this process slightly shifts toward lower temperatures for cross-linked MMT composites, which can be explained by less strong bonds between monomers and MMT in contrast to composites using temperature of organic decomposition and partial thermo-oxidation of the organic component in gels cross-linked by MMT compared to chemically cross-linked, their thermal stability remains relatively high—insignificant mass losses corresponding to the beginning of thermal destruction are observed at temperatures 310–330 °C, which is practically the same as for chemically cross-linked gels (310–335 °C).
The FTIR spectra of volatile products released during this complex process of organic decomposition (Fig. 6A) indicate the separation of amino groups (characteristic strong bands are observed at 966 and 930 cm−1 in the FTIR spectrum of volatile products), as well as separation of carbonyl group (the characteristic strong band at 1748 cm−1 and weak band at 1620 cm−1 is observed in the FTIR spectrum of volatile products). In addition, there is a destruction of the polymer backbone with the release of volatile products (characteristic weak bands at 2883 and 2978 cm−1 are observed in the FTIR spectrum, which correspond to symmetric and antisymmetric stretching vibrations of C-H bonds, respectively).
The thermal destruction process was carried out in an air atmosphere to simulate possible degradation mechanisms of synthesized hydrogels in natural conditions. In general, the volatile products released in the 3rd stage are given in Table 2, and scheme of the proposed mechanism of thermo-oxidation, defragmentation and destruction in the presence of oxygen is shown in Fig. 6B. Mass loss at the 3rd stage of thermal destruction significantly depends on the composition of hydrogels—for chemically cross-linked hydrogels, it ranges from 30.0 to 35.82%, for cross-linked with MMT it is from 22.4 to 25.2%, which is significantly less than for chemically cross-linked ones, and this corresponds to the ratio between the organic and inorganic components in composite hydrogels. The specified regularities correlate well with the results of the mentioned results of mass spectrometric study of the hydrogels thermolysis [61], where it is also noted that the thermal release of NH3 prevails in the temperature range of about 250 °C. This fragment can be split off from pAAm units when the latter form imide structures, which generally, is typical for the thermolysis of linear polyacrylamides [62]. Thus, the heating of pAAm and its cross-linked copolymer with acrylic acid leads to the destruction of polymer chains, and is accompanied by the release of fragments of CO, CO2, COOH and CONH2 groups. Note that the composition of the resulting fragments correlates with the composition of the co-polymeric hydrogel, which is subject to thermolysis, and if the cleaved amide group prevails in the thermal release products of the homo-polyacrylamide gel, then as the content of carboxyl groups increases, the amount of CO2 formed as a result of decarboxylation and cleaved carboxyl COOH groups increases. The release of CO2 during the 3rd stage of pAAm-based gels thermolysis can occur by one of the following mechanisms or their combination: (i) decarboxylation of COOH groups; (ii) cyclization with the participation of two neighboring carboxyl groups; or (iii) interaction of carbon atoms of the hydrocarbon chain during thermal oxidation. Regarding the mechanisms of thermal destruction in the gels studied by us in this paper, at the 3rd stage they may also be associated with the hydrolysis of amide groups present in the polymer with their polymer-analogous transformation to carboxyl ones and the formation of an ammonium cation [63] (Fig. 6B, Scheme). Confirmation of the above is the splitting during heating at the 2nd stage (200–270 °C) of the C = O stretching peak in the amide group of acrylamide (at 1649 cm−1) into two signals at 1625 and 1517 cm−1 belonging, respectively, to the amide and carboxyl group –COO− [64] in volatile reaction products.
In general, amide groups difficult interact with water molecules under neutral conditions and undergo hydrolysis. However, our FTIR data suggest that pAAm was partially hydrolyzed at 3d stage, and ammonia was formed during the reaction. According to literature data [65] additional water molecules, ammonia molecules, protonated water, and protonated ammonia can reduce the activation energy and catalyze the hydrolysis reaction of the amide group. This adequately explains the hydrolysis of pAAm under neutral conditions. The process of hydrolysis of pAAm amide groups can be schematically depicted in Fig. 6B.
The 4th peak is main peak of polymer thermal destruction for chemically cross-linked gels and composites cross-linked by MMT and it is observed at Tonset = 430–470 °C with a maximum at 562–620 and 504–511 °C, respectively. In case of samples with the addition of montmorillonite, this peak is noticeably narrower (the width of the peak corresponds to a smaller temperature range) compared to chemically cross-linked pAAm-based hydrogels. It may be due to the much greater homogeneity inherent in physically cross-linked hydrogels as opposed to chemically cross-linked ones. It should be noted that the heterogeneity of chemically cross-linked gels is one of their insurmountable disadvantages, caused by a significant difference in the values of the copolymerization constants inherent in the corresponding monomers and the cross-linking agent (the specified constant is much higher for MBAAm), as a result of which at the beginning of the structure formation the gel will have larger cross-linking than the one formed in the subsequent stages of gel synthesis. Thus, gel structure formation in the process of free-radical copolymerization of monomers with MBAAm proceeds unevenly, and on the contrary to physically cross-linked hydrogels with a uniform distribution of cross-links, real chemically cross-linked gels are always characterized by inhomogeneous cross-link density distribution, known as the spatial gel inhomogeneity [18]. The above causes a significant (in several times) increase in the thermal destruction peak width for chemically cross-linked hydrogels compared to physically cross-linked ones.
In this range, according to TG/DTG data, mass loss of 37.9–53 mass%. is observed (Table 2), which corresponds to the process of C and H atoms thermo-oxidation or burning of organic components of composites, and the FTIR spectra of the products released during this process (Figs. 7, 8 and S8) identify mainly peaks of H2O (in the range 3595–3735 cm−1), and CO2 (peaks centered at 2310 and 2360 cm−1), and CO (peaks centered at 2110 and 2185 cm−1) volatile products. This last high-temperature stage of thermal destruction corresponds to the combustion of the remained component of chemically cross-linked and physically cross-linked MMT hydrogels in the presence of air oxygen with the release of the corresponding volatile products CO, CO2 and H2O, which are reflected in the FTIR spectra by characteristic bands centered at 2110–2185, 2310–2360 and 3595–3735 cm−1, respectively. For H2O which is formed as a result of thermal oxidation of hydrogen atoms during polymer combustion, characteristic peaks are observed in the region 4000–3200 cm−1 range, when two very sharp and intense peaks occur at 3699 cm−1 and 3602 cm−1.
Based on the literature data [66], the former peak is assigned to the ν3 mode of H2O, while the latter peak is assigned to the ν1 mode of H2O. The absorption FTIR spectrum of CO2 shows the two regions typical of this molecule: at 3500–3800 cm−1 and 2200–2300 cm−1. A single ν3 antisymmetric stretching mode is observed at 2343 cm−1. The ν2 CO2 bending mode at 660 cm−1 is rather broad and undefined (Figs. 6 and 8). The ν2 bending mode is degenerated and can split into an in-plane bend at lower frequency and an out-of-plane bend at higher frequency upon a loss of linear symmetry. Additionally, a peak in the range of 2243–2250 cm−1 was observed, reflecting the emission of nitriles [67].
Laponite-cross-linked pAAm demonstrated a two-stage degradation process [60]. The first stage with slow mass loss was observed at 198–300 °C and corresponded to the moisture and low molecular weight pAAm loss. The second stage, with major mass loss at 288–500 °C, was attributed to the complete decomposition of the hydrogel. Marandy et al. developed pAAm-itaconic acid hydrogels containing different masses of laponite [67]. Three decomposition stages of the hydrogels were observed. For the composite with 1 g of laponite, the first was in the range from 28 °C to 198 °C, the second to 289 °C and the last to 600 °C with mass loss of 4.8, 17.5 and 46.7%, respectively. The composite with 4 g of laponite demonstrated higher thermal stability since the composite mass loss was 7% at 240 °C, 15% at 372 °C, and 25.3% at 600 °C. The MMT-cross-linked composite based on pAAm-plasticized starch with and without chitosan passed through one stage of thermal decomposition [38]. However, the main peak for the composite without and with chitosan was observed at 283.8 °C and 365.4 °C. Also, the peaks had different shapes: sharp for the hydrogel without chitosan, and broad for the one with chitosan.
Conclusions
The synthesis of a series of chemically cross-linked hydrogels based on pAAm, the copolymers of pAAm and acrylonitrile or acrylic acid, and a series of hydrogels based on the same monomers physically cross-linked with montmorillonite was carried out. It was determined that physically cross-linked hydrogels formed an intercalated/exfoliated structure in the case of pAAm-MMT and pAAm-AN-MMT gels and an intercalated structure in the case of pAAm-AA-MMT, confirmed by the increase in the basal spacing of [001] planes according XRD study and by the shift of the Si–O band (centered at 988 cm−1 for the pristine MMT) toward higher wave numbers for composites (1023–1026 cm−1) according to FTIR analyses. SEM images showed formation of a regular uniform cluster (periodic) structure in the form of MMT domains covered with the polymer layer. Comprehensive study of thermal properties of synthesized gels using TG, DTG, and DSC methods with FTIR analysis of volatile products of thermodestruction was carried out in an air atmosphere to simulate possible degradation mechanisms of synthesized hydrogels in natural conditions. It was found that the mechanisms of thermal destruction were similar for all used copolymers. Both in the case of chemically and physically cross-linked xerogels, thermal destruction includes four stages with the main mechanisms corresponding to the removal of water and weakly bound compounds in the temperature range up to 210 °C, defragmentation and separation of amino groups and the release of volatile ammonia compounds or primary amines up to 330 °C, organic decomposition in the range of 330–450 °C and burning of organics 450–1000 °C. This thermal behavior allows attributing the synthesized materials to relatively thermally stable.
The impact of MMT incorporation in pAAm-based gels on their thermal properties was as follows. Mass loss at the organic decomposition stage of thermal destruction for physically cross-linked gels (22.4–25.2%) was significantly less than for chemically cross-linked ones (30.0–35.8%) in the same temperature range, which corresponded to the ratio between organic and inorganic components in composite hydrogels. In addition, for physically cross-linked gels, the burning of organics peak width had a smaller temperature range compared to chemically cross-linked gels based on pAAm, which may be related to the greater homogeneity of them in comparison with chemically cross-linked ones.
The new synthesized hydrogels based on pAAm, and its copolymers with acrylonitrile or acrylic acid, physically cross-linked with the montmorillonite show good characteristics, such as better thermal stability and higher structural uniformity in comparison to those of chemically cross-linked materials. The used synthesis method limited the toxic effects of components applied in traditional chemical cross-linking method. The studies will be extended to obtaining entirely bio-safe hydrogels.
Abbreviations
- AA:
-
Acrylic acid
- AAm:
-
Acrylamide
- AN:
-
Acrylonitrile
- DSC:
-
Differential scanning calorimetry
- MBAAm:
-
N,N'-methylene-bis-acrylamide
- MMT:
-
Montmorillonite
- p(AAm):
-
Poly(acrylamide)
- p(AAm-AA):
-
Poly(acrylamide-co-acrylic acid)
- p(AAm-AA):
-
Poly(acrylamide-co-acrylonitrile)
- pAAm:
-
Poly(acrylamide) gel physically cross-linked with MMT
- pAAm-AA-MMT:
-
Poly(acrylamide-co-acrylic acid) gel physically cross-linked with MMT
- pAAm-AN-MMT:
-
Poly(acrylamide-co-acrylonitrile) gel physically cross-linked with MMT
- PPS:
-
Potassium persulfate
- TG/DTG:
-
Derivative thermogravimetry analyses
- FTIR:
-
Fourier transform infrared spectroscopy
- XRD:
-
X-ray diffractometry
References
Ramli RA. Slow release fertilizer hydrogels: a review. Polym Chem. 2019;10:6073–90.
Pakdel PM, Peighambardoust SJ. A review on acrylic based hydrogels and their applications in wastewater treatment. J Environ Manag. 2018;217:123–43.
Abousalman-Rezvani Z, Roghani-Mamaqani H, Riazid H, Abousalman-Rezvani O. Water treatment using stimuli-responsive polymers. Polym Chem. 2022;13:5940–64.
Sennakesavan G, Mostakhdemin M, Dkhar LK, Seyfoddin A, Fatihhi SJ. Acrylic acid/acrylamide based hydrogels and its properties—a review. Polym Degrad Stab. 2020;180: 109308.
Gils PS, Ray D, Mohanta P, Manavalan R, Sahoo PK. Designing of new acrylic based macroporous superabsorbent polymer hydrogel and its suitability for drug delivery. Int J Pharm Sci. 2009;1:43–54.
Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;107(7):1869–80.
Soppirnath KS, Aminabhavi TM. Water transport and drug release study from cross-linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release application. Eur J Pharm Biopharm. 2002;53:87–98.
Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345–60.
Das S, Dalei G. In situ forming dialdehyde xanthan gum-gelatin Schiff-base hydrogels as potent controlled release fertilizers. Sci Total Environ. 2023;875: 162660.
Kernosenko L, Samchenko K, Goncharuk O, Pasmurtseva N, Poltoratska T, Siryk O, Dziuba O, Mironov O, Szewczuk-Karpisz K. Polyacrylamide hydrogel enriched with amber for in vitro plant rooting. Plants. 2023;12(5):1196.
Lu H, Zhang Y, Tian T, Li X, Wu J, Yang H, Huang H. Preparation and properties of Sanxan gel based fertilizer for water retention and slow-release. Int J Biol Macromol. 2023;238: 124104.
Zhang X, Liu Y, Lu P, Zhang M. Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers. Green Process Synth. 2020;9(1):139–52.
Silversmith EF. Free-radical polymerization of acrylamide. J Chem Educ. 1992;69(9):763.
Saraydın D, Karadag E, Isıkver Y, Sahiner N, Güven O. The influence of preparation methods on the swelling and network properties of acrylamide hydrogels with crosslinkers. J Macromol Sci Part A. 2004;41:419–31.
Naghash HJ, Massah A, Erfan A. Free-radical crosslinking copolymerization of acrylamide and N, N′-methylenebis acrylamide by used Ce(IV)/polyethylene glycol and Ce(IV)/diethylmalonate redox initiator systems. Eur Polym J. 2002;38:147–50.
Mandal B, Ray SK. Swelling, diffusion, network parameters and adsorption properties of IPN hydrogel of chitosan and acrylic copolymer. Mater Sci Eng C. 2014;44:132–43.
Budtova TV, Budtov VP, Navard P, Frenkel SY. Rheological properties of highly swollen hydrogel suspensions. J Appl Polym Sci. 1994;52(6):721–6.
Orakdogen N, Kizilay MY, Okay O. Suppression of inhomogeneities in hydrogels formed by free-radical crosslinking copolymerization. Polymer. 2005;46:11407–15.
Bai Y, Liu Y, Yang K, Lang Y. Application and research prospect of functional polymer gels in oil and gas drilling and development engineering. Gels. 2023;9(5):413.
Puza F, Zheng Y, Han L, Xue L, Cui J. Physical entanglement hydrogels: ultrahigh water content but good toughness and stretchability. Polym Chem. 2020;11:2339–45.
Olad A, Zebhi H, Salari D, Mirmohseni A, Tabar AR. Water retention and slow release studies of a salep-based hydrogel nanocomposite reinforced with montmorillonite clay. New J Chem. 2018;42:2758–66.
Kuśtrowski P, Natkański P, Rokicińska A, Witek E. Polymer Hydrogel-Clay (Nano)Composites. In: Thakur VK, Thakur MK, editors. Polymer Gels: Science and Fundamentals. Singapore: Springer; 2018. p. 1–62.
Zhou Q, Li Y, Liu H, Li X. Tough nanocomposite hydrogel based on montmorillonite nanosheets/acrylic acid/acrylamide with copper removal properties. Coll Surf A Physicochem Eng Asp. 2020;598: 124836.
Qiu J, Du X, Komarneni S, Wang H, Cheng X, Du Z. Preparation of polyacrylamide-montmorillonite nanocomposite and its application in Cr(III) adsorption. J Appl Polym Sci. 2020;137(36):49065.
Ma P, Wang Z, Jiang Y, Huang Z, Xia L, Jiang J, Yuan F, Xia H, Zhang Y. Clay-based nanocomposite hydrogels with microstructures and sustained ozone release for antibacterial activity. Coll Surf A Physicochem Eng Asp. 2022;641: 128497.
Li P, Siddaramaiah, Kim NH, Yoo G-H, Lee J-H. Poly(acrylamide/laponite) nanocomposite hydrogels: Swelling and cationic dye adsorption properties. J Appl Polym Sci. 2009;111:1786–1798.
Wu J, Hill RJ. Laponite-doped poly(acrylic acid- co-acrylamide) hydrogels. ACS Appl Polym Mater. 2022;4(8):5927–40.
Goncharuk O, Samchenko Y, Kernosenko L, Korotych O, Poltoratska T, Pasmurtseva N, Oranska O, Sternik D, Mamyshev I. Thermoresponsive hydrogels physically crosslinked with magnetically modified LAPONITE® nanoparticles. Soft Matter. 2020;6:5689–701.
Fijałkowska G, Szewczuk-Karpisz K, Wiśniewska M. Chromium(VI) and lead(II) accumulation at the montmorillonite/aqueous solution interface in the presence of polyacrylamide containing quaternary amine groups. J Mol Liq. 2019;293: 111514.
Wiśniewska M, Fijałkowska G, Szewczuk-Karpisz K, Urban T, Nosal-Wiercińska A, Wójcik G. Comparison of adsorption affinity of anionic and cationic polyacrylamides for montmorillonite surface in the presence of chromium (VI) ions. Adsorption. 2019;25:41–50.
Haraguchi K, Li HJ, Matsuda K, Takehisa T, Elliott E. Mechanism of forming organic/inorganic network structures during in-situ free-radical polymerization in PNIPA−clay nanocomposite hydrogels. Macromolecules. 2005;38(8):3482–90.
Khan SA, Siddiqui MF, Khan TA. Ultrasonic-assisted synthesis of polyacrylamide/bentonite hydrogel nanocomposite for the sequestration of lead and cadmium from aqueous phase: equilibrium, kinetics and thermodynamic studies. Ultrason Sonochem. 2020;60: 104761.
Khan SA, Siddiqui MF, Khan TA. Synthesis of poly(methacrylic acid)/montmorillonite hydrogel nanocomposite for efficient adsorption of amoxicillin and diclofenac from aqueous environment: kinetic, isotherm, reusability, and thermodynamic investigations. ACS Omega. 2020;5:2843–55.
Ianchis R, Ninciuleanu CM, Gifu IC, Alexandrescu E, Somoghi R, Gabor AR, Preda S, Nistor CL, Nitu S, Petcu C, Icriverzi M, Florian PE, Roseanu AM. Novel hydrogel-advanced modified clay nanocomposites as possible vehicles for drug delivery and controlled release. Nanomaterials. 2017;7:443.
Chen M, Chen X, Zhang C, Cui B, Li Z, Zhao D, Wang Z. Kaolin-enhanced superabsorbent composites: synthesis, characterization and swelling behaviors. Polymer. 2021;13:1204.
El-Din HMN, Ibraheim DM. Biological applications of nanocomposite hydrogels prepared by gamma-radiation copolymerization of acrylic acid (AAc) onto plasticized starch (PLST)/montmorillonite clay (MMT)/chitosan (CS) blends. Int J Biol Macromol. 2021;192:151–60.
Natkański P, Białas A, Kuśtrowski P. The synthesis of poly(acrylic acid) -bentonite and polyacrylamide-bentonite composites for adsorption applications. Chemik. 2012;66:742–9.
Xiong L, Hu X, Liu X, Tong Z. Network chain density and relaxation of in situ synthesized polyacrylamide/hectorite clay nanocomposite hydrogels with ultrahigh tensibility. Polymer. 2008;49:5064–71.
Gao G, Du G, Sun Y, Fu J. Self-healable, tough, and ultrastretchable nanocomposite hydrogels based on reversible polyacrylamide/montmorillonite adsorption. ACS Mater Interfaces. 2015;7:5029–37.
Zhu M, Liu Y, Sun B, Zhang W, Liu X, Yu H, Zhang Y, Kuckling D, Adler H-JP. A novel highly resilient nanocomposite hydrogel with low hysteresis and ultrahigh elongation. Macromol Rapid Commun. 2006;27:1023–1028.
Xiong C, Wei F, Li W, Liu P, Wu Y, Dai M, Chen J. Mechanism of polyacrylamide hydrogel instability on high-temperature conditions. ACS Omega. 2018;3:10716–24.
Tongwa P, Nygaard R, Bai B. Evaluation of a nanocomposite hydrogel for water shut-off in enhanced oil recovery applications: Design, synthesis, and characterization. J Appl Polym Sci. 2013;128:787–94.
Zhang Q, Li X, Zhao Y, Chen L. Preparation and performance of nanocomposite hydrogels based on different clay. Appl Clay Sci. 2009;46(4):346–50.
Amiri S. Preparation and characterization of nanoclay-based (Na-mmt and bentonite) polyacrylamide hydrogels as water shut-off agent for enhanced oil recovery. Silicon. 2019;11:1193–203.
Shen J, Li N, Ye M. Preparation and characterization of dual-sensitive double network hydrogels with clay as a physical crosslinker. Appl Clay Sci. 2015;103:40–5.
Nguyen-Thai NU, Hong SC. Structural evolution of poly(acrylonitrile-co-itaconic acid) during thermal oxidative stabilization for carbon materials. Macromolecules. 2013;46:5882–9.
He ZP, Liu HC, Zhang S, Yang JL, Jiang C, Ji MW, Yu JL, Wang ML, Zhu CZ, Xu J. Investigation of the cyclization mechanism of poly(acrylonitrile-coethylenesulfonic acid) copolymer during thermal oxidative stabilization by in situ infrared spectroscopy. Ind Eng Chem Res. 2020;59:9519–31.
Kaşgöz H, Özgümüš S, Orbay M. Modified polyacrylamide hydrogels and their application in removal of heavy metal ions. Polymer. 2003;44:1785–93.
He Z, Liu H, Yang G, Jiang Ch, Ji M, Yu J, Wang M, Zhu C, Xu J. Cyclization mechanism and kinetics of poly(acrylonitrile-co-2-acrylamido-2-methylpropane sulfonic acid) copolymer. Polym Test. 2021;93: 106969.
Sundaraganesan GN, Joshua BD, Meganathan Ch, Sebastian S. Vibrational spectroscopic studies supported by HF/DFT calculations of 2,4,6-triaminopyrimidine. Indian J Chem, Sect. A. 2008;47A:821–29.
Patel HA, Somani RS, Bajaj HC, Jasra RV. Nanoclays for polymer nanocomposites, paints, inks, greases and cosmetics formulations, drug delivery vehicle and waste water treatment. Bull Mater Sci. 2006;29:133–45.
Madejová J. FTIR techniques in clay mineral studies. Vib Spectrosc. 2003;31:1–10.
Xi Y, Ding Z, He H, Frost RL. Infrared spectroscopy of organoclays synthesized with the surfactant octadecyltrimethylammonium bromide. Spectrochim Acta, Part A. 2005;61:515–25.
Ma Y, Zhu J, He H, Yuan P, Shen W, Liu D. Infrared investigation of organo-montmorillonites prepared from different surfactants. Spectrochim. Acta, Part A. 2010;76:122–29.
Ural N. The significance of scanning electron microscopy (SEM) analysis on the microstructure of improved clay: an overview. Open Geosci. 2021;13:1197–217.
Sapalidis AA, Katsaros FK, Kanellopoulos NK. PVA/montmorillonite nanocomposites: development and properties. in: cuppoletti j, editor. nanocomposites and polymers with analytical methods. London: IntechOpen. 2011;29–50.
Koç Z, Çelik M, Önal M, Sarikaya Y, Öner Y, Açik L. Study on the synthesis and properties of polyacrylamide/Na-montmorillonite nanocomposites. J Compos Mater. 2014;48(4):439–46.
Li P, Siddaramaiah, Kim NH, Yoo GH, Lee JH. Poly(acrylamide/laponite) nanocomposite hydrogels: Swelling and cationic dye adsorption properties. J Appl Polym Sci. 2009;111:1786–98.
Samchenko YM, Pokrovsky VA, Ulberg ZR, Chuiko AA. Mass spectrometric study of thermolysis of copolymer hydrogels based on acrylamide and acrylic acid. Rep Natl Acad Sci Ukr. 2004;4:142–7.
Leung WM, Axelson DE, Van Dyke JD. Thermal degradation of polyacrylamide and poly(acrylamide-co-acrylate). J Polym Sci Part A: Polym Chem. 1987;25:1825–46.
Xiong B, Loss RD, Shields D, Pawlik T, Hochreiter R, Zydney AL, Kumar M. Polyacrylamide degradation and its implications in environmental systems. Clean Water. 2018;1:17.
E Silva MESR, Dutra ER, Mano V, Machado JC. Preparation and thermal study of polymers derived from acrylamide. Polym Degrad Stab. 2000;67(3): 491–95.
Ma Q, Shuler PJ, CW Aften CW, Tang Y. Theoretical studies of hydrolysis and stability of polyacrylamide polymers. Polym Degrad Stab. 2015;121:69–77.
Della Ventura G, Bellatreccia F, Rossi P. The single-crystal, polarized-light, FTIR spectrum of stoppaniite, the Fe analogue of beryl. Phys Chem Minerals. 2007;34:727–31.
Van Dyke JD, Kasperski KL. Thermogravimetric study of polyacrylamide with evolved gas analysis. J Polym Sci Part A: Polym. Chem. 1993;31:1807–23.
Fu X, Yang Q, Y Zhang Y. Thermal decomposition behavior and mechanism study of cationic polyacrylamide. J Therm Anal Calorim. 2021;146:1371–81.
Marandi GB, Baharloui M, Kurdtabar M, Sharabian LM, Mojarrad MA. Hydrogel with high laponite content as nanoclay: swelling and cationic dye adsorption properties. Res Chem Intermed. 2015;41:7043–58.
Acknowledgements
The study was partially financed by National Science Centre, Poland (Olena Goncharuk 2022/01/3/NZ9/00042; Olena Siryk 2022/01/3/NZ9/00043) under the support for Ukrainian scientists.
Author information
Authors and Affiliations
Contributions
Dariusz Sternik contributed to conceptualization, investigation (DSC and SEM studies), formal analysis, resources; writing—review & editing; Katarzyna Szewczuk-Karpisz contributed to supervision, visualization, resources, project administration; Olena Siryk contributed to investigations (synthesis and XRD); Yurii Samchenko contributed to conceptualization, visualization, formal analysis, resources; Anna Deryło-Marczewska contributed to supervision, visualization, resources, validation; Lyudmila Kernosenko contributed to formal analysis, visualization, validation; Eugen Pakhlov contributed to investigation (FTIR spectroscopy) and resources; and Olena Goncharuk contributed to formal analysis, visualization, validation, writing—review & editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sternik, D., Szewczuk-Karpisz, K., Siryk, O. et al. Structure and thermal properties of acrylic copolymer gels: effect of composition and cross-linking method. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13430-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13430-y