Abstract
The study examined building materials containing asbestos, which have been considered hazardous waste for several years. Samples were taken from various places in Poland. The chemical composition was examined using chemical analysis, the mineralogical phases were identified using X-ray diffraction, and the structure was identified using scanning electron microscopy, taking into account energy-dispersive spectroscopy. Thermal tests of the samples were performed using thermal analysis, thermogravimetric measurements and high-temperature microscopy. Additionally, changes that occurred in the microstructure were determined using mercury porosimetry and infrared spectroscopy. All the above research methods were used to characterise the properties of cement–asbestos materials, which were also subjected to isothermal thermal treatment at a temperature of 1100 °C for 4 h. The results proved that the material after thermal treatment undergoes significant structural changes. The thermal decomposition process of cement–asbestos involves dehydration, dehydroxylation and then recrystallisation to new stable crystalline phases but in the context of asbestos, we are dealing here with the so-called phenomenon of pseudomorphosis. Knowledge about the thermal properties of asbestos materials can provide us with data on how the material undergoes significant structural changes, thanks to which it will be possible to use neutralised cement–asbestos waste as possible safe materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asbestos is a group of natural minerals with special chemical and physical properties. Asbestos minerals can be divided into mineralogy groups like the serpentine and amphiboles [1,2,3]. The main property of all asbestos materials is their fibrous form, with fibres of the thinnest diameter in nature. In the nineteenth and twentieth centuries, asbestos fibres were especially valued and were used in the manufacture of more than 3000 various products [4, 5]. Mainly popular assortments containing asbestos fibres were cement–asbestos products for the construction industry [2, 6], which used over 80% of the asbestos mined worldwide [7]. For example, according to the estimation, ∼ 90% of asbestos brought to Poland was exploited to form cement–asbestos sheets. The quantity of asbestos-containing products in Poland is estimated to reach roughly 15.5 million tonnes, most of them (∼ 15.0 million tonnes) are cement–asbestos products [8].
Currently, the danger resulting from the use of asbestos materials is known, especially their carcinogenic properties (group 1 in the International Agency for Research and Cancer (IARC)) [9]. Knowledge of asbestos-related diseases is widely described in the science literature [10,11,12,13]. This is the main reason why asbestos materials have been recognised as hazardous waste in some countries and their disposal has been confined to special hazardous waste landfills. However, the use and production of asbestos is forbidden only in nearly 70 countries [14,15,16,17,18,19]. A similar solution also operates in Poland, but its implementation is not satisfactory [20,21,22,23]. By applicable regulations and the obligation towards the European Union, in 2009 a long-term programme for the elimination of asbestos and asbestos-containing materials was adopted in Poland, although since 1997 there had already been an official prohibition of using and producing asbestos materials. According to the programme assumptions, materials containing asbestos must be removed and stored in hazardous waste landfills.
Analysis of the situation in Poland shows that the above programme is being implemented too slowly [23]. Moreover, it should be strongly emphasised that this is not the final solution. The fibrous structure is still present and the risk of secondary pollution of the surroundings is high. Furthermore, by the priorities of waste management and the Waste Act [24, 25], more effective methods of waste disposal should be used first, and landfilling should be treated as the last solution. Such an approach would be following the new circular economy action plan (CEAP) approved by the European Commission [26]. It fits into the assumptions of the European Green Deal, which is a programme related to sustainable development in Europe.
It is necessary to look for a method to destroy the asbestos structure so that it ceases to pose a threat to living organisms and the environment. A possible solution to the problem is the complete annihilation of asbestos by destroying its fibrous structure and converting it into new mineral phases that can be reused, which is essential considering the non-renewable and shrinking natural resources. According to the specialist literature and review articles focusing on asbestos disposal methods [27,28,29,30,31], there are many methods of neutralising asbestos, e.g. chemical [32,33,34,35,36,37,38], biological [39,40,41], mechanochemical [42,43,44,45] and broadly understood thermal [46,47,48,49,50]. The last seems to be the most prospective, due to the lack of harmful post-process reagents and the relatively short process time as well as the flexibility to treat wastes of various types.
One of the proposed methods could be the classical thermal degradation process. As a result of the thermal processing of asbestos, chemically bound water is released, which in turn leads to a change in the crystal structure [51]. In consequence, the creation of new mineral phases occur. In the case of chrysotile asbestos, its thermal decomposition contributes to the transformation to forsterite (Mg2SiO4) and/or enstatite (MgSiO3). The detailed mechanism is described elsewhere [52,53,54,55]. A similar mechanism should be expected in the case of cement–asbestos products. However, in this case, a cementitious matrix is in the system, which may affect the entire process. During the thermal treatment of asbestos, phase transition was observed and the phase composition was different compared to raw asbestos minerals and asbestos-containing waste [56,57,58,59,60].
The research in this study aimed to determine the structural and phase transformations of cement–asbestos materials while heating them to high temperature. Understanding the thermal decomposition process of asbestos-cement waste opens up new application possibilities and the use of the resulting secondary raw material in future applications.
Experimental
In this piece of research, five samples of cement–asbestos roofing materials selected from various locations in Poland were studied. Materials for the research came from two regions of Poland, i.e. from the Silesian and Lesser Poland voivodeships. Three of them were obtained from the Mikołów district (BUJ, NOW, HEL), the fourth from the Oświęcim district (BRZ) and the fifth from the city of Dąbrowa Górnicza (DG). All samples come from private owners, as corrugated cement–asbestos boards removed from roofs. The exact time of their use, the date of disassembly, or the time and conditions of ageing after dismantling are not known.
To avoid the randomness of the samples, for each material, a laboratory sample was taken for testing in the following way. Primary samples were taken from each board in the form of broken fragments of several centimetres from the four corners and the middle of the tested board. This material was used for the determination of the main physical properties of these cement–asbestos samples. The hydrostatic weighing method determined the samples’ open porosity (OP), apparent density (AD) and water absorbability (WA). Each parameter was determined at least in three parallel samples. Then, these larger fragments were crushed and combined into one sample, which was ground in the next step. To attain homogeneous representative samples the coning and quartering method was applied. These raw cement–asbestos samples were instrumentally studied by X-ray fluorescence (XRF), differential thermal analysis (DTA), thermogravimetric analysis (TG), X-ray diffraction (XRD), infrared spectroscopy (FTIR), mercury porosimetry, scanning electron microscopy (SEM) and high-temperature optical microscopy. Moreover, all cement–asbestos samples were isothermally fired at 1100 °C for 4 h and additional studies were performed (XRD, FTIR).
The chemical analysis of the raw cement–asbestos samples was performed by X-ray fluorescence (XRF; PANalytical, Almelo, Netherlands), using a Panalytical Magix PW-2424 spectrometer, based on fused cast-bead method described in PN-EN ISO 12677:2011 standard. The chemical analysis was supplemented by loss on ignition (L.O.I.) value measured by isothermal calcination at 1025 °C.
The phase compositions of raw samples and the materials obtained after thermal treatment were determined by powder X-ray diffraction (XRD; PANalytical, Almelo, Netherlands). The analyses were conducted using a PANalytical X’pert Pro diffractometer (CuKα radiation, Ni filter, 40 kV, 30 mA, X’Celerator detector).
Thermal analysis (DTA and TG/DTG) with evolved gas analysis was performed on the STA 409 PC Luxx apparatus (Netzsch, Selb, Germany) which was coupled with the quadrupole mass spectrometer QMS 403 C Aëolos. About 100 mg of cement–asbestos sample was placed on an alumina crucible and heated from ambient temperature to 1450 °C at a rate of 20 °C min−1 in an air atmosphere with a 10 mL min−1 rate.
IR spectra were measured on a Nicolet 6700 FTIR spectrophotometer by the ATR method (Thermo Fischer Scientific, Waltham, USA), while mercury porosimetry was performed using the Autopore 9500 mercury porosimeter (Micromeritics Instruments, Norcross, USA), which was used for this purpose, allowed testing the porosity and grain size distribution within a range of 0.006–450 μm.
Microstructure analysis was performed using Mira 3 electron microscope (Tescan, Brno, Czech Republic) equipped with an energy-dispersive spectrometer (EDS) system with AZtec Automated software (Oxford Instruments, Abingdon, United Kingdom). The analysis was performed at an accelerating voltage of 15 kV in the backscattered electron mode (BSE) for image formation. The measurements were carried out on the samples covered by a conductive layer of graphite by using a Quorum Q150R ES device (Quorum Technologies, Laughton, United Kingdom).
So-called characteristic temperatures (beginning of sintering, softening and melting point) were determined using high-temperature microscopy (Leitz, Wetzlar, Germany). The ground powder material with acetone was pressed manually into a 3 mm cube. The sample was heated in an air atmosphere to a temperature of 1500 °C at a rate of 7 °C min−1.
Results and discussion
The conditions of use of the materials and their exposure to weather conditions varied. There is no detailed information on how long the product has been operating in outdoor conditions. In terms of oxides (Table 1), their chemical composition was typically compared to that described in the literature [61]. The main components of all cement–asbestos samples were CaO and SiO2, which come mainly from cementitious matrix. The content of silicon oxide varied from 18.6 mass% for the DG sample to 21.4 mass% for the NOW sample. On the contrary, the calcium oxide content is more differentiated and changed from 36.5 mass% for the NOW sample to ~ 46 mass% for the DG cement–asbestos sample. Because the materials were ageing for a long time counted in tens of years, a significant loss in ignition values (20–25 mass%) are also observed mainly related to the thermal decomposition of the ageing products of the cement matrix.
In addition to these main chemical components of raw cement–asbestos samples, secondary ingredients like aluminium oxide (from 3.6 to 4.5 mass%), iron oxide (from 2.5 to 3.3 mass%), sulphur oxide (from 1.3 to 2.2 mass%), and magnesium oxide (from 3.5 to 9.9 mass%) were also identified. The presence of the latter may indirectly indicate the presence of asbestos, since the main asbestos mineral used in construction products such as cement–asbestos roofing material was chrysotile, i.e. hydrated magnesium silicate, Mg3(OH)4Si2O5. An increased concentration of MgO, especially for the NOW sample, may indicate a significant degradation and ageing of this sample, due to leaching of the cement matrix and simultaneous enrichment of the cement–asbestos product with asbestos. This phenomenon was confirmed when we compared open porosity and water absorption values (Fig. 1). For this sample, the values of determined above physical properties were the highest, while the apparent density was the lowest (ca. 1.70 g cm−3 vs 1.90–1.95 g cm−3 for other samples).
All tested cement–asbestos materials were characterised by a large variation in the open porosity (OP) range and water absorption (WA), the values of which vary from ~ 15 to 30% in the case of OP and changed from ~ 7.0 to 17.5% for WA, respectively. Moreover, mercury porosimetry showed a high total share of pores with diameters below 1 µm ranging from 83.4 to 90.4% (Fig. 2). Different pore size distributions were also observed. The highest amount of pores with a pore size diameter of around 0.1 μm was identified for samples NOW, HEL, and BRZ. In turn, the DG sample was characterised by the highest amount of pores greater than 0.5–1 μm.
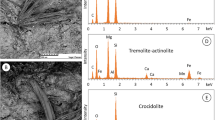
For all cement–asbestos materials, the presence of characteristic fibrous structure in the material was found (Fig. 3), which confirmed the presence of asbestos fibres in the samples. The surface of the asbestos–cement boards was relatively sleek and compact, where packets of the asbestos fibres were well bonded in the cement matrix. In the fracture of the samples, the asbestos fibres were visible. Chemical analysis by SEM–EDS shows that the asbestos fibres consist mainly of O, Mg, and Si, indicating the chrysotile type of asbestos.
The qualitative phase composition of all cement–asbestos materials measured by the XRD method was similar. The diffraction reflexes of the cementitious matrix dominate on the X-ray diffraction patterns (Fig. 4) of all tested samples. The main crystal components identified in all samples were portlandite (Ca(OH)2) and calcite (CaCO3). These cement–asbestos samples contained also some minor mineral phases such as gypsum (CaSO4·2H2O), ettringite (Ca6Al2(SO4)3(OH)12·26H2O), larnite (Ca2SiO4), hatrurite (Ca3SiO5) and katoite (Ca3Al2(SiO4)3−x(OH)4x x = 1.5–3). Their presence is not surprising, and they are often identified in this type of material where cement was used as a mineral binder and also in cement–asbestos wastes [61].
These are the main mineral phases that are created during the setting, hardening, and ageing of the cement paste [61,62,63]. The hydration process of cement binders has been studied for many years [61, 64]. Portland cement reacts with water producing various hydrated compounds from which calcium silicate hydrates (the CSH phase) and portlandite are the main. Calcium aluminates presented in cement clinker react with water and gypsum (added as a setting time regulator) producing among others ettringite. Due to the low degree of crystallinity of the CSH phase, these phases are difficult to identify and are responsible for a high mass fraction of the amorphous component. In our case, hydrogarnets of the katoite-hibschite series were detected. With time, as a result of the weathering and carbonation process of the cement matrix, calcite was formed. Furthermore, unreacted clinker phases such as larnite and hatrurite (C2S and C3S in cement notation, respectively) were also identified.
For all samples, their XRD patterns show the presence of chrysotile as the main asbestos mineral. This is confirmed by the characteristic narrow and intense two diffraction reflexes at 2Theta c.a. 12° and 24°. This is not surprising, as chrysotile is the main asbestos mineral mined on an industrial scale. Chrysotile corresponds to 90–95% of total asbestos mined in the world [2, 18] and now is only one type of asbestos mineral that is still mined and applied [65]. It is worth noting that for the NOW sample intensity of these reflexes was the biggest, which may confirm a significant degree of weathering and ageing in this sample.
For samples BUJ and HEL, additional visible XRD reflexes at ~ 10.5 2Theta were measured. The appearance of this reflex indicates the presence of minerals from the amphibole group. In the case of this study, crocidolite asbestos was also identified in samples. Because Poland did not have its usable asbestos deposits and the entire amount was imported into the country over the years, it can be assumed that the presence of crocidolite fibres is due to the deliberate addition or replacement of chrysotile, rather than as primary impurities of chrysotile. Interestingly, unlike the Italian experience [61], no aggregates and fillers were identified in domestic asbestos-cement products.
The thermal decomposition of all the examined cement–asbestos samples was nearly identical (Figs. 5–7). Only in the case of the NOW sample the obtained curves, especially DTA, was different. In all curves obtained, the effects resulting from the decomposition of the cementitious matrix dominated. Between 100 and 200 °C the endothermic peak associated with the mass change is visible on all DTA curves. For all samples, this phenomenon can be connected with the loss of water from the CSH phase [66]. This effect was stronger for the BRZ sample, which was characterised by the lowest value of loss on ignition (Table 1), i.e. relatively the lowest degree of ageing, which was also visible for the physical properties (Fig. 1). The endothermic peak near 500 °C is associated with the presence of portlandite (Ca(OH)2) and its thermal decomposition (Ca(OH)2 → CaO + H2O). This effect was the strongest for the NOW sample and the lowest for the BUJ cement–asbestos sample. This is in agreement with X-ray results (Fig. 4), during which the highest intensity of portlandite reflex was recorded for the NOW sample. It is worth noting here that for the NOW sample at a temperature around 200 °C a slight inflection in the DTA curve was also visible. This effect was connected with the mass change which was visible on the DTG curve. This effect was also observed (to a lesser extent) for BUJ and BRZ samples. It probably comes from the thermal decomposition of gypsum and its dehydration by hemihydrate to anhydrite, CaSO4·2H2O → CaSO4·1/2H2O → CaSO4 + 2H2O. According to the literature [67, 68], this reaction occurs in the temperature range of 110–200 °C. On DTG curves of all cement–asbestos samples near 400 °C small peaks were observed. It may be related to the brucite decomposition. According to the literature data, brucite decomposes at 200–500 °C [69]. The size of the mass loss on the DTG curve corresponds to the MgO content in the samples. The smallest mass loss was recorded for BUJ and DG samples containing 3.48 mass% and 4.54 mass% MgO, respectively. The largest loss in mass was recorded in the NOW sample, which contained the highest amount (9.89 mass%) of MgO.
At higher temperature (in the range of 600–850 °C) two overlapping endothermic peaks were observed. The first one is weak, in a wide temperature range and the second one has a characteristic asymmetric shape and maximum at ~ 820 °C. The wide temperature range of the first effect as well as the presence of water vapour and carbon dioxide in evolved gas analysis (Supplementary material) may indicate the thermal decomposition of calcium carbonate (CaCO3) with different degrees of crystallinity of calcite [70] or other cementitious phases. Stepkowska et al. [71] showed that the residual water evaporated from the aged cement pastes in the range of 500 and 700 °C, probably by dehydroxylation of the jennite-like compound (9CaO·6SiO2·11H2O). The second peak with an asymmetric shape is typical for the decomposition of calcium carbonate [72, 73], calcite: CaCO3 → CaO + CO2. In the temperature range of 1200–1400 °C, especially on TG/DTG curves of all the cement–asbestos samples, a small mass loss (~ 2 mass%) was visible. Investigation of the released gas proved the presence of sulphur as SO2, and the effect comes from the thermal decomposition of calcium sulphate.
Due to the relatively low asbestos content in the cement–asbestos material, characteristic thermal effects resulting from the decomposition of the asbestos minerals were not observed. The typical endothermic effect related to the dehydroxylation of chrysotile asbestos and the further exothermic crystallisation to forsterite [52,53,54,55] was masked by the thermal effect connected with the breakdown of the cementitious matrix. Only in the case of the NOW sample, where the amount of asbestos fibres was the highest as a result of the leaching and ageing of the sample, on the DTA curve at ~ 800 °C small exothermic effect without mass change was noticed.
The thermal behaviour of the cement–asbestos samples was confirmed by infra-red spectroscopy (Fig. 8). The characteristic broad band between 3200 and 3700 cm−1 (including small double peaks at 3640–3680 cm−1) attributed to OH stretching vibrations (νO–H) of asbestos, as well as a characteristic carbonate band at about 1400 cm−1 (νC=O), were disappeared after the thermal treatment (see Fig. 8 vs. Fig. 9). This confirms the decomposition of asbestos minerals [74,75,76].
The thermal decomposition of cement–asbestos samples was additionally confirmed by the XRD analysis. New reflexes can be seen on the XRD patterns (Fig. 10) of all samples obtained after thermal treatment by isothermal calcination. They show the creation mainly of larnite (Ca2SiO4) and merwinite (Ca3Mg(SiO4)2), especially in the case of the NOW sample. Probably, due to the higher amount of magnesium in the system for the NOW sample, during heating the latter was created as a result of a different location in the CaO–MgO–SiO2 ternary phase diagram. Moreover, there are also new mineral phases such as ternesite (Ca5(SiO4)2SO4), ye’elimite (Ca4Al6O12(SO4)), and brownmillerite (Ca2(Al,Fe)2O5). These phases show binding properties and may be used as components of different mineral binders [77,78,79,80,81]. The presence of periclase (MgO) indirectly indicates the thermal decomposition of chrysotile asbestos. The results show that CaCO3 present in the material promotes the formation of calcium-silicate compounds and accelerates decomposition reactions [82]. In this instance, the decomposition of chrysotile with calcite leads to the formation of silicate phases according to the following reaction: Mg3Si2O5(OH)4 + 4CaCO3 → 2Ca2SiO4 + 3MgO + 2H2O + 4CO2 [82], and not to the transformation into forsterite, which that might be expected. On all XRD patterns of material obtained after thermal treatment (Fig. 10), a strong main reflex coming from periclase at ~ 43° 2Theta was observed. The qualitative character of the thermal decomposition of the considered asbestos-cement wastes shows a very similar course.
The behaviour of cement–asbestos wastes during thermal load was also checked using a high-temperature microscope. The results (Fig. 11) were similar for all tested materials and showed that significant changes occurred at a temperature above 1100 °C. All tested cement–asbestos materials begin to sinter at a temperature of about 1060 °C, which is observable as the deviation of the curve from the base curve. In the temperature range 1100–1200 °C the liquid phase probably formed. Their amount systematically increased, which was indicated by the beginning of intense sintering, and then softening of the sample as a rapid change in the cross-section area in the temperature between 1200 and 1300 °C. The melting point of materials shifted towards higher temperatures. The measurement indicates a significant increase in the quantity of the liquid phase in the cement–asbestos samples above the temperature of 1420 °C.
The SEM photographs of the selected materials after thermal treatment are presented in Figs. 12 and 13. Figure 12 shows the presence of pseudomorphic transformation of the asbestos fibres. It is known that after thermal decomposition of asbestos minerals, the newly formed mineral phases preserve the original external fibrous habit although a complete modification of the structure at the molecular scale has occurred. Due to this phenomenon named as pseudomorphosis the original asbestos fibre irreversibly loses its strength. They are more brittle and can be fractured perpendicular to the fibre axis, so they are easily fragmented into spherical grains of new silicate minerals [83, 84]. SEM characterisation of crushed material (Fig. 13) showed an easily grindable material with isolated, single spherical and rounded grains. No fibrous forms that would indicate the presence of the residual pseudomorphs after asbestos fibres were detected. The maximal diameter of obtained grains does not exceed 2 μm, practically more of them were below 1 μm.
Research has shown the possibility of transforming waste containing asbestos into new material without asbestos and its fibrous form. Therefore, the thermal method can be indicated as an interesting method of dealing with asbestos waste on an industrial scale. Unlike disposal in landfills, this would be a valuable alternative in solving the broadly understood asbestos problem. As a result of the thermal treatment, it is possible to obtain a valuable secondary raw material with a wide range of applications. This material could be used in the production of hydraulic mineral binders [85,86,87], as an additive in the production of construction ceramics [88, 89], geopolymers [90, 91] and as an additive to mortars [92] or for the production of glass fibres [93]. It can be also useful in the reduction of raw material exploitation [94]. Although thermal treatment seems to be the most expensive due to the energy consumption, this method has a lot of implementation potential mentioned above and the cost is not as high as it initially seems. As the literature indicates [29, 95], the energy consumption for thermal treatment methods varies in the range of 0.5–1.5 kWh per kg of treated asbestos waste. Notwithstanding, this cost seems to be passable. Considering the average price of electricity in Poland in 2023 (0.2 €/kWh), the potential cost of thermal treatment for one tonne of asbestos waste is in the range of 100–300 €. At this moment, the average cost of asbestos waste storage at the landfill connected with the disassembly or transport cost is around 250–350 € per tonne. Therefore, it can be initially estimated that this method is feasible for implementation on a larger scale.
Conclusions
Thermal treatment is one of the available techniques for the utilisation of asbestos waste, including cement–asbestos materials. The research in this article was intended to characterise the physical and thermal properties of cement–asbestos materials. Five samples of the material were obtained from two voivodeships of Poland and characterised in the raw state and after heating at a temperature of 1100 °C. The analysed cement–asbestos samples were tested by different instrumental methods (XRD, XRF, DTA/TG, SEM/EDS, FTIR and mercury porosimetry). Several differences were noticed between all tested asbestos materials. In particular, this applies to chemical composition and main physical properties (water absorption, open porosity and apparent density of raw samples). These differences may result from the conditions of use of the materials and their exposure to atmospheric factors. In turn, there are no significant differences among the groups of cement–asbestos materials used under thermal treatment. In each case, the thermal decomposition takes place similarly. Up to a temperature of 800 °C, there was a significant decrease in the mass of all tested samples by approximately 25%, and this is related to the processes of dehydration (100–200 °C), dehydroxylation and thermal decomposition of minerals (brucite decomposes at 200 °C to 500 °C, portlandite decomposition near 500 °C, jennite-like compound at 500–700 °C, asbestos at 600–700 °C, calcite up to 800 °C). In the temperature range of 1200–1400 °C, only a slight mass loss of approximately 2% was observed due to the decomposition of sulphates. All samples have similar values of characteristic temperatures, i.e. sintering temperature around 1060 °C, softening point between 1200 and 1300 °C and melting point above 1400 °C. The results show that during treatment at high-temperature remarkable changes occur in the material. Consequently, they permit further reuse of thermally treated asbestos wastes. Through this process, the fibrous structure of asbestos minerals was fully changed although the fibre habit is preserved (pseudomorphosis). The obtained materials were characterised by high grindability and can be reused in various further applications. Due to the presence of mineral phases exhibiting binding properties, the search for directions related to the production of mineral binders seems to be purposeful and desirable.
Data availability
The experimental data that support the findings of this study are available in RepOD repository with the identifier https://doi.org/10.18150/XCDVVX
References
Ciullo PA. Industrial minerals and their uses—a handbook and formulary. 1st ed. New York: William Andrew Publishing; 1996.
Virta RL. Mineral commodity profiles—Asbestos. Circular 1255-KK. Reston: U.S. Geological Survey; 2005.
Gualtieri AF. Mineral fibers: crystal chemistry, chemical-physical properties, biological interaction and toxicity. London: European Mineralogical Union; 2017.
Harris LV, Kahwa IA. Asbestos: old foe in 21st century developing countries. Sci Total Environ. 2003;307:1–9. https://doi.org/10.1016/S0048-9697(02)00504-1.
Bensted J, Smith JR. Asbestos legacy impacts on future prospects. Cem Wapno Beton. 2011;3:161–6.
Howe-Grant M. Kirk-Othmer encyclopedia of chemical technology. 4th ed. Hoboken: Wiley; 1997.
Dyczek J. Exploitation and disposal of asbestos-containing products. Mater Bud. 2006;411:46–52.
Council of Ministers of the Republic of Poland. Program for the removal of asbestos and asbestos-containing products used in Poland. Warsaw: Polish Government; 2002.
International Agency for Research on Cancer (IARC). IARC monographs on the evaluation of carcinogenic to humans supplement 7, overall evaluations of carcinogenicity: an updating of IARC Monographs. Lyon: International Agency for Research on Cancer; 1973. pp. 1–42.
Musk AW, de Klerk N, Reid A, Hui J, Franklin P, Brims F. Asbestos-related diseases. Int J Tuberc Lung Dis. 2020;24(6):562–7. https://doi.org/10.5588/ijtld.19.0645.
Kang D, Myung MS, Kim YK, Kim JE. Systematic review of the effects of asbestos exposure on the risk of cancer between children and adults. Ann Occup Environ Med. 2013;25:10. https://doi.org/10.1186/2052-4374-25-10.
Cheng TJ, More SL, Maddaloni MA, Fung ES. Evaluation of potential gastrointestinal carcinogenicity associated with the ingestion of asbestos. Rev Environ Health. 2021;26(1):15–26. https://doi.org/10.1515/reveh-2020-0061.
Kwak K, Kang D, Paek D. Environmental exposure to asbestos and the risk of lung cancer: a systematic review and meta-analysis. Occup Environ Med. 2022;79:207–14. https://doi.org/10.1136/oemed-2020-107222.
The International Ban Asbestos Secretariat (IBAS). Available online http://www.ibasecretariat.org/index.htm. Accessed 14 Jul 2023.
Silvestri S. Managing asbestos in Italy: twenty years after the ban. N Solut. 2012;22:489–96. https://doi.org/10.2190/NS.22.4.g.
Marsili D, Angelini A, Bruno C, Corfiati M, Marinaccio A, Silvestri S, Zona A, Comba P. Asbestos ban in Italy: a major milestone, not the final cut. Int J Environ Res Public Health. 2017;14(11):1379. https://doi.org/10.3390/ijerph14111379.
Paglietti F, Malinconico S, della Staffa BC, Bellagamba S, De Simone P. Classification and management of asbestos-containing waste: European legislation and the Italian experience. Waste Manag. 2016;50:130–50. https://doi.org/10.1016/j.wasman.2016.02.014.
Frank AL, Joshi TK. The global spread of asbestos. Ann Glob Health. 2014;80:257–62. https://doi.org/10.1016/j.aogh.2014.09.016.
Li J, Dong Q, Yu K, Liu L. Asbestos and asbestos waste management in the Asian-Pacific region: trends, challenges and solutions. J Clean Prod. 2014;81:218–26. https://doi.org/10.1016/j.jclepro.2014.06.022.
Supreme Audit Office. Information about the control results from the implementation of the Programme for Asbestos Abatement in Poland 2009–2032. Warsaw: Supreme Audit Office; 2015. (in Polish).
Szułczyńska D. Determinants of the perception of asbestos. Saf Fire Tech. 2019;53:144–61. https://doi.org/10.12845/sft.51.3.2019.9.
Szymańska D, Lewandowska A. Disposal of asbestos and products containing asbestos in Poland. J Mater Cycles Waste Manag. 2019;21:345–55. https://doi.org/10.1007/s10163-018-0796-4.
Kusiorowski R, Lipowska B, Kujawa M, Gerle A. Problem of asbestos-containing wastes in Poland. Clean Waste Syst. 2023;4:1000085. https://doi.org/10.1016/j.clwas.2023.1000085.
Regulation of the Minister of the Environment of 27 September 2001 on the waste catalogue. (Journal of Laws No. 112, item 1206). (in Polish).
Waste Act. (Journal of Laws of 4 January 2018, item 21). (in Polish).
EU Parliament, Document 52020DC0098, A new circular economy action plan for a cleaner and more competitive Europe, COM/2020/98 final. Accessed 14 Jul 2023.
Gualtieri AF. Recycling asbestos-containing material (ACM) from construction and demolition waste (CDW). In: Pacheco-Torgal F, Tam VWY, Labrincha JA, Ding Y, de Brito J, editors. Handbook of recycled concrete and demolition waste, vol. 47. Cambridge: Woodhead Publishing; 2013. p. 500–25. https://doi.org/10.1533/9780857096906.4.500.2013.
Spasiano D, Pirozzi F. Treatments of asbestos containing wastes. J Environ Manag. 2017;204:82–91. https://doi.org/10.1016/j.jenvman.2017.08.038.
Paolini V, Tomassetti L, Segreto M, Borin D, Liotta F, Torre M, Petracchini F. Asbestos treatment technologies. J Mater Cycles Waste Manag. 2019;21:205–26. https://doi.org/10.1007/s10163-018-0793-7.
Gualtieri AF, Giacobbe C, Sardisco L, Saraceno M, Gualtieri ML, Lusvardi G, Cavenati C, Zanatto I. Recycling of the product of thermal inertization of cement–asbestos for various industrial applications. Waste Manag. 2011;31:91–100. https://doi.org/10.1016/j.wasman.2010.07.006.
European Commission. Directorate-general for environment. In: Akelytė R, Chiabrando F, Camboni M, Ledda C, Vencovsky D, Butera S, Dünger O, editors. Study on asbestos waste management practices and treatment technologies. Luxembourg: Publications Office of the European Union; 2024. https://doi.org/10.2779/251640.
Sugama T, Sabatini R, Petrakis L. Decomposition of chrysotile asbestos by fluorosulfonic acid. Ind Eng Chem Res. 1998;37:79–88. https://doi.org/10.1021/ie9702744.
Turci F, Tomatis M, Mantegna S, Cravotto G, Fubini B. A new approach to the decontamination of asbestos-polluted waters by treatment with oxalic acid under power ultrasound. Ultrason Sonoch. 2008;15:420–7. https://doi.org/10.1016/j.ultsonch.2007.08.007.
Pawełczyk A, Božek F, Grabas K, Chęcmanowski J. Chemical elimination of the harmful properties of asbestos from military facilities. Waste Manag. 2016;61:377–85. https://doi.org/10.1016/j.wasman.2016.11.041.
Nam SN, Jeong S, Lim H. Thermochemical destruction of asbestos-containing roofing slate and the feasibility of using recycled waste sulfuric acid. J Hazard Mater. 2014;265:151–7. https://doi.org/10.1016/j.jhazmat.2013.11.004.
Rozalen M, Huertas FJ. Comparative effect of chrysotile leaching in nitric, sulfuric and oxalic acids at room temperature. Chem Geol. 2013;352:134–42. https://doi.org/10.1016/j.chemgeo.2013.06.004.
Turci F, Tomatis M, Mantegna S, Cravotto G, Fubini B. The combination of oxalic acid with power ultrasound fully degrades chrysotile asbestos fibres. J Environ Monit. 2007;9:1064–6. https://doi.org/10.1039/B709571F.
Yanagisawa K, Kozawa T, Onda A, Kanazawa M, Shinohara J, Takanami T, Shiraishi M. A novel decomposition technique of friable asbestos by CHClF2- decomposed acidic gas. J Hazard Mater. 2009;163:593–9. https://doi.org/10.1016/j.jhazmat.2008.07.017.
Favero-Longo SE, Turci F, Tomatis M, Castelli D, Bonfante P, Hochella MF, Piervittori R, Fubini B. Chrysotile asbestos is progressively converted into a non-fibrous amorphous material by the chelating action of lichen metabolites. J Environ Monit. 2005;7:764–6. https://doi.org/10.1039/B507569F.
David SR, Ihiawakrim D, Regis R, Geoffroy VA. Efficiency of pyoverdines in iron removal from flocking asbestos waste: an innovative bacterial bioremediation strategy. J Hazard Mater. 2020;394: 122532. https://doi.org/10.1016/j.jhazmat.2020.122532.
Borges R, Klaic R, Farinas CS, Ribeiro C. Biological treatment of asbestos cement wastes by Aspergillus niger and Acidithiobacillus thiooxidans. App Clay Sci. 2022;216: 106375. https://doi.org/10.1016/j.clay.2021.106375.
Plescia P, Gizzi D, Benedetti S, Camilucci L, Fanizza C, De Simone P, Paglietti F. Mechanochemical treatment to recycling asbestos-containing waste. Waste Manag. 2003;23:209–18. https://doi.org/10.1016/S0956-053X(02)00156-3.
Colangelo F, Cioffi R, Lavorgna M, Verdolotti L, De Stefano L. Treatment and recycling of asbestos-cement containing waste. J Hazard Mater. 2011;195:391–7. https://doi.org/10.1016/j.jhazmat.2011.08.057.
Bloise A, Kusiorowski R, Gualtieri AF. The effect of grinding on tremolite asbestos and anthophyllite asbestos. Minerals. 2018;8(7):274. https://doi.org/10.3390/min8070274.
Bloise A, Catalano M, Gualtieri AF. Effect of grinding on chrysotile, amosite and crocidolite and implications for thermal treatment. Minerals. 2018;8(4):135. https://doi.org/10.3390/min8040135.
Bloise A, Catalano M, Barrese E, Gualtieri AF, Bursi Gandolfi N, Capella S, Belluso E. TG/DSC study of the thermal behaviour of hazardous mineral fibres. J Therm Anal Calorim. 2016;123:2225–39. https://doi.org/10.1007/s10973-015-4939-8.
Boccaccini DN, Leonelli L, Rivasi MR, Romagnoli M, Veronesi P, Pellacani GC, Boccaccini AR. Recycling of microwave inertised asbestos containing waste in refractory materials. J Eur Ceram Soc. 2007;27:1855–8. https://doi.org/10.1016/j.jeurceramsoc.2006.05.003.
Dellisanti F, Rossi PL, Valdrè G. Remediation of asbestos containing materials by Joule heating vitrification performed in a pre-pilot apparatus. Int J Miner Process. 2009;91:61–7. https://doi.org/10.1016/j.minpro.2008.12.001.
Gualtieri AF, Lassinantti Gualtieri M, Tonelli M. In situ ESEM study of the thermal decomposition of chrysotile asbestos in view of safe recycling of the transformation product. J Hazard Mater. 2008;156:260–6. https://doi.org/10.1016/j.jhazmat.2007.12.016.
Inaba T, Nagano M, Endo M. Investigation of plasma treatment for hazardous wastes such as fly ash and asbestos. Electr Eng Jpn. 1999;126:73–82. https://doi.org/10.1002/(SICI)1520-6416(199902)126:3%3c73::AID-EEJ8%3e3.0.CO;2-J.
Bloise A. On the thermal breakdown of tremolite: a new method for distinguishing between asbestos and non-asbestos tremolite samples. J Mater Sci. 2023;58:8779–95. https://doi.org/10.1007/s10853-023-08595-0.
Zaremba T, Krząkała A, Piotrowski J, Garczorz D. Study on the thermal decomposition of chrysotile asbestos. J Therm Anal Calorim. 2010;101:479–85. https://doi.org/10.1007/s10973-010-0819-4.
Bloise A, Kusiorowski R, Lassinantti Gualtieri M, Gualtieri AF. Thermal behaviour of mineral fibres. In: Gualtieri AF, editor. Mineral fibres: crystal chemistry, chemical-physical properties, biological interaction and toxicity. London: European Mineralogical Union and the Mineralogical Society of Great Britain & Ireland; 2017. p. 215–60. https://doi.org/10.1180/emu-notes.18.7.
MacKenzie KJD, Meinhold RH. Thermal reactions of chrysotile revisited: a 29Si and 25Mg MAS NMR study. Am Miner. 1994;79:43–50.
Cattaneo A, Gualtieri AF, Artioli G. Kinetic study of the dehydroxylation of chrysotile asbestos with temperature by in situ XRPD. Phys Chem Miner. 2003;30:177–83. https://doi.org/10.1007/s00269-003-0298-2.
Gualtieri AF, Tartaglia A. Thermal decomposition of asbestos and recycling in traditional ceramics. J Eur Ceram Soc. 2000;20:1409–18. https://doi.org/10.1016/S0955-2219(99)00290-3.
Gualtieri AF, Cavenati C, Zanatto I, Meloni M, Elmi G, Gualtieri ML. The transformation sequence of cement–asbestos slates up to 1200°C and safe recycling of the reaction product in stoneware tile mixtures. J Hazard Mater. 2008;152:563–70. https://doi.org/10.1016/j.jhazmat.2007.07.037.
Kusiorowski R, Zaremba T, Piotrowski J, Adamek J. Thermal decomposition of different types of asbestos. J Therm Anal Calorim. 2012;109:693–704. https://doi.org/10.1007/s10973-012-2222-9.
Kusiorowski R, Zaremba T, Piotrowski J, Gerle A. Thermal decomposition of asbestos-containing materials. J Therm Anal Calorim. 2013;113:179–88. https://doi.org/10.1007/s10973-013-3038-4.
Witek J, Kusiorowski R. Neutralization of cement–asbestos waste by melting in an arc-resistance furnace. Waste Manag. 2017;69:336–45. https://doi.org/10.1016/j.wasman.2017.08.017.
Viani A, Gualtieri AF, Secco M, Peruzzo L, Artioli G, Cruciani G. Crystal chemistry of cement–asbestos. Am Miner. 2013;98:1095–105. https://doi.org/10.2138/am.2013.4347.
Taylor HFW. Cement chemistry. London: Academic Press; 1997.
Scrivener K, Nonat A. Hydration of cementitious materials, present and future. Cem Concr Res. 2011;41:651–65. https://doi.org/10.1016/j.cemconres.2011.03.026.
Bullard JW, Jennings HM, Livingston RA, Nonat A, Scherer GW, Schweitzer JS, Scrivener KL, Thomas JJ. Mechanisms of cement hydration. Cem Concr Res. 2011;41:1208–23. https://doi.org/10.1016/j.cemconres.2010.09.011.
Flanagan DM. Mineral commodity summaries. Asbestos 2021. Reston: U.S. Geological Survey; 2022.
Zhang Q, Ye G. Dehydration kinetics of Portland cement paste at high temperature. J Therm Anal Calorim. 2012;110:153–8. https://doi.org/10.1007/s10973-012-2303-9.
Engbrecht DC, Hirschfeld DA. Thermal analysis of calcium sulfate dihydrate sources used to manufacture gypsum wallboard. Thermochim Acta. 2016;639:173–85. https://doi.org/10.1016/j.tca.2016.07.021.
Dantas HF, Mendes RAS, Pinho RD, Soledade LEB, Paskocimas CA, Lira BB, Schwartz MOE, Souza AG, Santos IMG. Characterization of gypsum using TMDSC. J Therm Anal Calorim. 2007;87:691–5. https://doi.org/10.1007/s10973-006-7733-9.
Wang JA, Novaro O, Bokhimi X, Lopez T, Gomez R, Navarrete J, Llanos ME, Lopez-Salinas E. Characterizations of the thermal decomposition of brucite prepared by sol-gel technique for synthesis of nanocrystalline MgO. Mater Lett. 1998;35:317–23. https://doi.org/10.1016/S0167-577X(97)00273-5.
Dias CMR, Cincotto MA, Savastano H, John VM. Long-term aging of fiber-cement corrugated sheets—the effect of carbonation, leaching and acid rain. Cem Concr Compos. 2008;30:255–65. https://doi.org/10.1016/j.cemconcomp.2007.11.001.
Stepkowska ET, Blanes JM, Real C, Perez-Rodriguez JL. Hydration products in two aged cement pastes. Thermochim Acta. 2004;420:79–87. https://doi.org/10.1016/j.cemconcomp.2007.11.001.
Halikia I, Zoumpoulakis L, Christodoulou E, Prattis D. Kinetic study of the thermal decomposition of calcium carbonate by isothermal methods of analysis. Eur J Miner Process Environ Protect. 2001;1:89–102.
Chattaraj BD, Dutta SN, Iyengar MS. Studies on the thermal decomposition of calcium carbonate in the presence of alkali salts (Na2CO3, K2CO3 and NaCl). J Therm Anal. 1973;5:43–9. https://doi.org/10.1007/BF01914472.
Iwaszko J, Zawada A, Lubas M. Influence of high-energy milling on structure and microstructure of asbestos-cement materials. J Mol Struct. 2018;1155:51–7. https://doi.org/10.1016/j.molstruct.2017.10.104.
Iwaszko J, Zawada A, Przerada I, Lubas M. Structural and microstructural aspects of asbestos-cement waste vitrification. Spectrochim Acta A Mol Biomol Spectrosc. 2018;195:95–102. https://doi.org/10.1016/j.saa.2018.01.053.
Iwaszko J. Making asbestos-cement products safe using heat treatment. Case Stud Constr Mater. 2019;10: e00221. https://doi.org/10.1016/j.cscm.2019.e00221.
Shen Y, Wang P, Chen X, Zhang W, Qian J. Synthesis, characterisation and hydration of ternesite. Constr Build Mater. 2021;270: 121392. https://doi.org/10.1016/j.conbuildmat.2020.121392.
Hanein T, Galan I, Glasser FP, Skalamprinos S, Elhoweris A, Imbabi MS, Bannerman MN. Stability of ternesite and the production at scale of ternesite-based clinkers. Cem Concr Res. 2017;98:91–100. https://doi.org/10.1016/j.cemconres.2017.04.010.
Gołaszewska M, Klemczak B, Gołaszewski J. Thermal properties of calcium sulphoaluminate cement as an alternative to ordinary Portland cement. Materials. 2021;14:7011. https://doi.org/10.3390/ma14227011.
Julphunthong P, Joyklad P. Utilization of several industrial wastes as raw material for calcium sulfoaluminate cement. Materials. 2019;12:3319. https://doi.org/10.3390/ma12203319.
Kusiorowski R, Lipowska B, Gerle A. Synthesis of ye’elimite from anthropogenic waste. Minerals. 2023;13:137. https://doi.org/10.3390/min13020137.
Belardi C, Piga L. Influence of calcium carbonate on the decomposition of asbestos contained in end-of-life products. Thermochim Acta. 2013;573:220–8. https://doi.org/10.1016/j.tca.2013.08.019.
Bloise A. Thermal behaviour of actinolite asbestos. J Mater Sci. 2019;54:11784–95. https://doi.org/10.1007/s10853-019-03738-8.
Giacobbe C, Gualtieri AF, Quartieri S, Rinaudo C, Allegrina M, Andreozzi GB. Spectroscopic study of the product of thermal transformation on chrysotile-asbestos containing materials. Eur J Miner. 2010;22:535–46. https://doi.org/10.1127/0935-1221/2010/0022-2038.
Yvon Y, Sharrock P. Characterization of thermochemical inactivation of asbestos containing wastes and recycling the mineral residues in cement products. Waste Biomass Valor. 2011;2:169–81. https://doi.org/10.1007/s12649-011-9063-9.
Carneiro GO, Santos TA, Simonelli G, Ribeiro DV, Cilla MS, Dias CMR. Thermal treatment optimization of asbestos cement waste (ACW) potentializing its use as alternative binder. J Clean Prod. 2021;320:128801. https://doi.org/10.1016/j.jclepro.2021.128801.
Santos TA, Cilla MS. Use of asbestos cement tile waste (ACW) as mineralizer in the production of Portland cement with low CO2 emission and lower energy consumption. J Clean Prod. 2022;335:130061. https://doi.org/10.1016/j.jclepro.2021.130061.
Kusiorowski R, Zaremba T, Piotrowski J. The potential use of cement–asbestos waste in the ceramic masses destined for sintered wall clay brick manufacture. Ceram Int. 2014;40:11995–2002. https://doi.org/10.1016/j.ceramint.2014.04.037.
Kusiorowski R, Zaremba T, Piotrowski J, Podwórny J. Utilisation of cement–asbestos wastes by thermal treatment and the potential possibility use of obtained product for the clinker bricks manufacture. J Mater Sci. 2015;50:6757–67. https://doi.org/10.1007/s10853-015-9231-6.
Santana HA, Amorim Júnior NS, Carneiro GO, Ribeiro DV, Cilla MS, Dias CMR. Asbestos-cement wastes as supplementary precursors of NaOH-activated binders. Constr Build Mater. 2023;364: 129921. https://doi.org/10.1016/j.conbuildmat.2022.129921.
Carneiro GO, Santana HA, Ribeiro DV, Cilla MS, Dias CMR. One-part alkali-activated binder produced from inertized asbestos cement waste. J Clean Prod. 2022;367: 132966. https://doi.org/10.1016/j.jclepro.2022.132966.
Capitani G, Dalpiaz M, Vergani F, Campanale F, Conconi R, Odorizzi S. Recycling thermally deactivated asbestos cement in mortar: a possible route towards a rapid conclusion of the “asbestos problem.” J Environ Manag. 2024;355: 120507. https://doi.org/10.1016/j.jenvman.2024.120507.
Iwaszko J, Lubas M, Sitarz M, Zajemska M, Nowak A. Production of vitrified material from hazardous asbestos-cement waste and CRT glass cullet. J Clean Prod. 2021;317: 128345. https://doi.org/10.1016/j.jclepro.2021.128345.
Marian NM, Giorgetti G, Magrini C, Capitani GC, Galimberti L, Cavallo A, Salvini R, Vanneschi C, Viti C. From hazardous asbestos containing wastes (ACW) to new secondary raw material through a new sustainable inertization process: a multimethodological mineralogical study. J Hazard Mater. 2021;413: 125419. https://doi.org/10.1016/j.jhazmat.2021.125419.
Boldrin A, Maresca A, Fauser P, Sanderson H, Astrup TF. Waste containing asbestos and other environmentally problematic substances: Characterization, risks and management. Danish Environmental Protection Agency: Miljøprojekter No. 2216; 2022.
Acknowledgements
This research was funded in whole by the National Science Centre, Poland under “Sonata 17” grant number UMO-2021/43/D/ST5/00356. For Open Access, the author has applied a CC-BY public copyright licence to any Author Accepted Manuscript (AAM) version arising from this submission. The authors would like to thank Elwira Cieślińska and Maria Pyka for their help in preparing samples for testing.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by RK, AG, MK, JA, AŚ. The first draft of the manuscript was written by RK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest or competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kusiorowski, R., Gerle, A., Kujawa, M. et al. Characterisation of asbestos-containing wastes by thermal analysis. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13312-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13312-3