Abstract
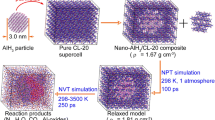
Based on a brand-new and excellent layered model of mixed explosives, the thermolysis process of HMX/CL-20 mixed explosives at different temperatures was studied by reactive molecular dynamics. The thermolysis mechanism was revealed on the atomic scale, and some gratifying new discoveries were obtained. Among them, the activation energy of the mixture in two stages was lower than that of the pure HMX system in different degrees, and CL-20 would promote the thermolysis of HMX. The analysis of the initial reaction path showed that a large amount of NO2 brought by CL-20 and OH generated by HNO2 decomposition would be the important factors of this phenomenon. The direct reaction between HMX and CL-20 also changed with the change of temperature. Furthermore, the addition of CL-20 would increase the number of final products such as H2O, N2, H2. The analysis of bond number brought a new conclusion. A large number of C atoms in the mixture were in clusters, and the larger clusters would not decompose even at the high temperature of 3500 K. The “lower” external temperature cannot drove the clusters composed of so many C atoms to change. To put it briefly, CL-20 would speed up HMX’s thermolysis and alter the system’s overall response mechanism.

















Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Muravyev NV, Fershtat L, Zhang Q. Synthesis, design and development of energetic materials: Quo Vadis. Chem Eng J. 2024;486:150410. https://doi.org/10.1016/j.cej.2024.150410.
Zhong K, Zhang CY. Review of the decomposition and energy release mechanisms of novel energetic materials. Chem Eng J. 2024;483:149202. https://doi.org/10.1016/j.cej.2024.149202.
Hao LN, Liu XQ, Zhai DD, Qiu L, Ma CM, Ma P, Jiang JC. Theoretical studies on the performance of HMX with different energetic groups. ACS Omega. 2020;5(46):29922–34. https://doi.org/10.1021/acsomega.0c04237.
Singh H, Jahagirdar N, Banerjee S. Sonochemically assisted synthesis of nano HMX. Def Technol. 2019;15(6):837–43. https://doi.org/10.3969/j.issn.2214-9147.2019.06.002.
Xu JJ, Cheng KM, Zhang HB, Hao XF, Xu XM, Sun J, Tian Y. Effect of metal ion impurities on the crystallization and thermal properties of HMX. J Therm Anal Calorim. 2022;147:6109–18. https://doi.org/10.1007/s10973-021-10850-y.
Viswanath JV, Venugopal KJ, Rao NVS, Venkataraman A. An overview on importance, synthetic strategies and studies of 2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaazaisowurtzitane (HNIW). Def Technol. 2016;12(5):401–18. https://doi.org/10.1016/j.dt.2016.05.002.
Nair UR, Sivabalan R, Gore GM, Geetha M, Asthana SN, Singh H. Hexanitrohexaazaisowurtzitane (CL-20) and CL-20-based formulations. Combust Explos Shock Waves. 2005;41:121–32. https://doi.org/10.1007/s10573-005-0014-2.
Kalman J, Essel J. Influence of particle size on the combustion of CL-20/HTPB propellants. Propellants Explos Pyrotech. 2017;42(11):1261–7. https://doi.org/10.1002/prep.201700137.
Liang WT, Sun XY, Wang H, Wang JK, Sui ZS, Ren HC, Dai RC, Zheng XX, Wang ZP, Duan XH, Zhang ZM. Isothermal structural evolution of CL-20/HMX cocrystals under slow roasting at 190 °C. Phys Chem Chem Phys. 2023;25(23):15756–66. https://doi.org/10.1039/D3CP01084H.
Jangid SK, Talawar MB, Singh MK, Nath T, Sinha RK. Experimental studies on advanced sheet explosive formulations based on 2, 4, 6, 8, 10, 12-Hexanitro-2, 4, 6, 8, 10, 12-hexaazaisowurtzitane (CL-20) and Hydroxyl Terminated polybutadiene (HTPB), and comparison with a RDX-based system. Cent Eur J Energ Mater. 2016;13(1):135–47. https://doi.org/10.22211/cejem/64968.
Zhao XT, Li JZ, Quan SX, Fu XL, Meng SQ, Jiang LP, Fan XZ. Study on the effect of solvent on cocrystallization of CL-20 and HMX through theoretical calculations and experiments. RSC Adv. 2022;12(33):21255–63. https://doi.org/10.1039/d2ra03730k.
Hang GY, Yu WL, Wang T, Wang JT. Theoretical investigations on structures, stability, energetic performance, sensitivity, and mechanical properties of CL-20/TNT/HMX cocrystal explosives by molecular dynamics simulation. J Mol Model. 2019;25(1):10. https://doi.org/10.1007/s00894-018-3887-1.
Yan QL, Zeman S, Elbeih A. Recent advances in thermal analysis and stability evaluation of insensitive plastic bonded explosives (PBXs). Thermochim Acta. 2012;537:1–12. https://doi.org/10.1016/j.tca.2012.03.009.
Herrmannsdörfer D, Stierstorfer J, Klapötke TM. Solubility behaviour of CL-20 and HMX in organic solvents and solvates of CL-20. Energ Mater Front. 2021;2(1):51–61. https://doi.org/10.1016/j.enmf.2021.01.004.
Sun SH, Zhang HB, Liu Y, Xu JJ, Huang SL, Wang SM, Sun J. Transitions from separately crystallized CL-20 and HMX to CL-20/HMX cocrystal based on solvent media. Cryst Growth Des. 2018;18(1):77–84. https://doi.org/10.1021/acs.cgd.7b00775.
Liu ZC, Wu Q, Zhu WH, Xiao HM. Insights into the roles of two constituents CL-20 and HMX in the CL-20: HMX cocrystal at high pressure: a DFT-D study. RSC Adv. 2015;5(43):34216–25. https://doi.org/10.1039/c5ra01829c.
Zhang J, Guo W. The role of electric field on decomposition of CL-20/HMX cocrystal: a reactive molecular dynamics study. J Comput Chem. 2021;42(31):2202–12. https://doi.org/10.1002/jcc.26748.
Xue X, Ma Y, Zeng Q, Zhang CY. Initial decay mechanism of the heated CL-20/HMX cocrystal: a case of the cocrystal mediating the thermal stability of the two pure components. J Phys Chem C. 2017;121(9):4899–908. https://doi.org/10.1021/acs.jpcc.7b00698.
Bolton O, Simke LR, Pagoria PF, Matzger AJ. High power explosive with good sensitivity: a 2:1 cocrystal of CL-20: HMX. Cryst Growth Des. 2012;12(9):4311–4. https://doi.org/10.1021/cg3010882.
Anderson SR, am Ende DJ, Salan JS, Samuels P. Preparation of an energetic‐energetic cocrystal using resonant acoustic mixing. Propellants Explos Pyrotech. 2014;39(5):637–40. https://doi.org/10.1002/prep.201400092.
Song CK, An CW, Zhang YR, Ye BY, Li HQ, Wang JY. Insensitive CL-20/Cyclotetramethylenetetranitramine (HMX) co-crystals with high performance by ultrasonic in solvent. Int J Energ Mater Chem Propul. 2017;16(4):321. https://doi.org/10.1615/IntJEnergeticMaterialsChemProp.2018022517.
Zhou S, Pang A, Tang G. Crystal transition behaviors of CL-20 in polyether solid propellants plasticized by nitrate esters containing both HMX and CL-20. New J Chem. 2017;41(24):15064–71. https://doi.org/10.1039/c7nj03309e.
Ding L, Zhao FQ, Liu ZR. Thermal decomposition of CL-20/HMX mixed system. J Solid Rocket Technol. 2008;02:164–7. https://doi.org/10.3969/j.issn.1006-2793.2008.02.016.
Xie HM, Zhu WH. Thermal decomposition mechanisms of the energetic benzotrifuroxan: 1, 3,3-trinitroazetidine cocrystal using ab initio molecular dynamics simulations. J Chin Chem Soc. 2020;67(2):218–26. https://doi.org/10.1002/jccs.201900169.
Yuan XF, Zhang SH, Gou RJ, Huang Y, Bai H, Guo QJ. ReaxFF/lg molecular dynamics study on thermolysis mechanism of NTO/HTPB plastic bonded explosive. Comput Theor Chem. 2022;1215:113834. https://doi.org/10.1016/j.comptc.2022.113834.
Parepalli P, Nguyen YT, Sen O, Hardin DB, Molek CD, Welle EJ, Udaykumar HS. Multi-scale modeling of shock initiation of a pressed energetic material III: effect of Arrhenius chemical kinetic rates on macro-scale shock sensitivity. J Appl Phys. 2024;135:085106. https://doi.org/10.1063/5.0187735.
Long Y, Chen J. Theoretical study of the interfacial force-field and thermodynamic properties for HMX-Estane mixture explosives. Propellants Explos Pyrotech. 2024;49:e202300265. https://doi.org/10.1002/prep.202300265.
Yuan XF, Huang Y, Zhang SH, Gou RJ, Zhu SF, Guo QJ. Multi-aspect simulation insight on thermolysis mechanism and interaction of NTO/HMX-based plastic-bonded explosives: a new conception of the mixed explosive model. Phys Chem Chem Phys. 2023;25(31):20951–68. https://doi.org/10.1039/d3cp00494e.
Zhao XQ, Shi NC. The crystal structure of ε-Hexnitrohexazaisowurtzitane. Chin Sci Bull. 1995;40(23):2158–60. https://doi.org/10.1360/csb1995-40-23-2158.
Bolotina NB, Hardie MJ, Speer JRL, Pinkerton AA. Energetic materials: variable-temperature crystal structures of γ-and ∊-HNIW polymorphs. J Appl Crystallogr. 2004;37(5):808–14. https://doi.org/10.1107/S0021889804017832.
Xu XJ, Xiao JJ, Huang H, Li JS, Xiao HM. Molecular dynamic simulations on the structures and properties of ɛ-CL-20 (0 0 1)/F2314 PBX. J Hazard Mater. 2010;175(1–3):423–8. https://doi.org/10.1016/j.jhazmat.2009.10.023.
Xiao JJ, Wang WR, Chen J, Ji GF, Zhu W, Xiao HM. Study on structure, sensitivity and mechanical properties of HMX and HMX-based PBXs with molecular dynamics simulation. Comput Theor Chem. 2012;999:21–7. https://doi.org/10.1016/j.comptc.2012.08.006.
Bai H, Gou RJ, Chen MH, Zhang SH, Chen YH. ReaxFF/lg molecular dynamics study on thermal decomposition mechanism of 1-methyl-2, 4, 5-trinitroimidazole. Comput Theor Chem. 2022;1209:113594. https://doi.org/10.1016/j.comptc.2022.113594.
Jiang J, Jiang QL, Chen YH, Hao WZ, Liu YB, Zhang SH. ReaxFF MD simulations of thermolysis mechanism of 2, 6-diamino-3, 5-dinitropyrazine-1-oxidated. Comput Theor Chem. 2020;1185:112891. https://doi.org/10.1016/j.comptc.2020.112891.
Yim WL, Liu Z. Application of ab initio molecular dynamics for a priori elucidation of the mechanism in unimolecular decomposition: the case of 5-nitro-2, 4-dihydro-3 H-1, 2, 4-triazol-3-one (NTO). J Am Chem Soc. 2001;123(10):2243–50. https://doi.org/10.1021/ja0019023.
Botcher TR, Beardall DJ, Wight CA, Fan LM, Burkey TJ. Thermal decomposition mechanism of NTO. J Phys Chem. 1996;100(21):8802–6. https://doi.org/10.1021/jp952984y.
Sviatenko LK, Gorb L, Leszczynski J. NTO degradation by direct photolysis: DFT study. Struct Chem. 2023;34(1):23–31. https://doi.org/10.1007/s11224-022-01923-1.
Ren CX, Li XX, Guo L. Reaction mechanisms in the thermal decomposition of CL-20 revealed by ReaxFF molecular dynamics simulations. Acta Phys Chim Sin. 2018;34(10):1151–62. https://doi.org/10.3866/PKU.WHXB201802261.
Cao ZM, Zong WJ, Zhang JJ, Xiao CW, Huang JH, Liu W, Wei ZY, He CL. Desensitising effect of water film on initial decomposition of HMX crystal under nano-cutting conditions by ReaxFF MD simulations. Mol Simul. 2020;46(7):530–40. https://doi.org/10.1080/08927022.2020.1736289.
Li YQ, Li B, Zhang D, Xie LF. Theoretical studies on CL-20/HMX based energetic composites under external electric field. Chem Phys Lett. 2021;778:138806. https://doi.org/10.1016/j.cplett.2021.138806.
Liu LC, Liu Y, Zybin SV, Sun H, Goddard WA III. ReaxFF-lg: correction of the ReaxFF reactive force field for London dispersion, with applications to the equations of state for energetic materials. J Phys Chem A. 2011;115(40):11016–22. https://doi.org/10.1021/jp201599t.
Yuan XF, Guo QJ, Zhang SH, Gou RJ, Huang Y. Comprehensive study on thermal decomposition mechanism and interaction of 3-Nitro-1, 2, 4-Triazol-5-One/Poly-3-nitromethyl-3-methyloxetane plastic bonded explosives. J Anal Appl Pyrolysis. 2022;168:105753. https://doi.org/10.1016/j.jaap.2022.105753.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340:53–68. https://doi.org/10.1016/S0040-6031(99)00253-1.
Chen F, Jia FS, Chen Y, Li TH, Guo GQ, Dong L. Reaction molecular dynamics simulation of thermal decomposition of HMX at high temperature. J At Mol Phys. 2023;2025(02):111–6.
Li X, Jin B, Luo L, Chu S, Peng R. Study on the isothermal decomposition of CL-20/HMX co-crystal by microcalorimetry. Thermochim Acta. 2020;690:178665. https://doi.org/10.1016/j.tca.2020.178665.
Zhou TT, Huang FL. Effects of defects on thermal decomposition of HMX via ReaxFF molecular dynamics simulations. J Phys Chem B. 2011;115(2):278–87. https://doi.org/10.1021/jp105805w.
Zhang L, Zybin SV, Van Duin ACT, Dasgupta S, Goddard WA III, Kober EM. Carbon cluster formation during thermal decomposition of octahydro-1, 3, 5, 7- tetranitro-1, 3, 5, 7-tetrazocine and 1, 3, 5-triamino-2, 4, 6-trinitrobenzene high explosives from ReaxFF reactive molecular dynamics simulations. J Phys Chem A. 2009;113(40):10619–40. https://doi.org/10.1021/jp901353a.
Huo XY, Wang FF, Niu LL, Gou RJ, Zhang CY. Clustering rooting for the high heat resistance of some CHNO energetic materials. FirePhysChem. 2021;1(1):8–20. https://doi.org/10.1016/j.fpc.2021.02.007.
Zhang YP, Yang Z, Li QK, He YH. Carbon-rich clusters and graphite-like structure formation during early detonation of 2, 4, 6-Trinitrotoluene (TNT) via molecular dynamics simulation. Acta Chim Sinica. 2018;76(7):556–63. https://doi.org/10.6023/A18040153.
Funding
This work was supported by Fundamental Research Program of Shanxi Province (No. 20210302123055).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. Tianhao Li and Ling Dong performed molecular dynamics simulations. Guoqi Guo and Fang Chen processed and analyzed the calculated data, and wrote the first draft of the paper. All authors revised and approved the final version of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, G., Chen, F., Li, T. et al. Comprehensive atomic insight into the whole process of thermolysis of HMX/CL-20 mixed explosives based on a brand-new layered model of mixed explosives. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13222-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13222-4