Abstract
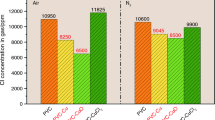
13X zeolite–calcium oxide (CaO) composite capture agent application in the pyrolysis recovery of polyvinyl chloride (PVC)-based waste cables was investigated in this research to elucidate the agent’s efficiency in capturing chlorine (Cl) and carbon (C) during pyrolysis. The 13X zeolite–CaO mixture is subjected to separate pyrolysis of the PVC-based cable outer sheath in experimental furnaces of simulated solar-powered and thermogravimetry. Subsequently, a range of spectroscopic techniques are employed to quantitatively analyze the gaseous and solid products resulting from the pyrolysis process. Through X-ray fluorescence, total carbon, and other analyses, the research outcomes demonstrated that the capture agent exhibits superior Cl and C capture performance compared to using a single agent for plastic pyrolysis. The yield of Cl element in the solid residue has increased significantly by 130–140% under the presence of optimized composite capture agent. By determining the dynamic changes of gaseous and solid products during the cable outer sheath pyrolysis, the influence of the capture agent on the redistribution and transformation mechanisms of Cl and C elements was identified, and it was found that contaminating volatiles were well immobilized in the solid residue. This research provides a new solution for efficient Cl and C capture in the pyrolysis recovery of waste cables and determines the fixation and transformation mechanisms of Cl and C elements by the composite capture agent, indicating a potential for feasible and sustainable resource utilization of waste cables.











Similar content being viewed by others
References
Zou Y, Li Y, Bourbigot S, Zhang J, Guo Y, Li K, et al. Determination of solid-phase reaction mechanism and chlorine migration behavior of co-pyrolyzing PVC-CaCO3 based polymer using temperature-dependent FTIR and XRD analysis. Polym Degrad Stabil. 2021. https://doi.org/10.1016/j.polymdegradstab.2021.109741.
Tongamp W, Zhang Q, Shoko M, Saito F. Generation of hydrogen from polyvinyl chloride by milling and heating with CaO and Ni(OH)2. J Hazard Mater. 2009;167(1–3):1002–6. https://doi.org/10.1016/j.jhazmat.2009.01.076.
Wang Z, Xie T, Ning X, Liu Y, Wang J. Thermal degradation kinetics study of polyvinyl chloride (PVC) sheath for new and aged cables. Waste Manag. 2019;99:146–53. https://doi.org/10.1016/j.wasman.2019.08.042.
Micoli L, Bagnasco G, Turco M. HCl removal from biogas for feeding MCFCs: adsorption on microporous materials. Int J Hydrog Energy. 2013;38(1):447–52. https://doi.org/10.1016/j.ijhydene.2012.09.102.
Suresh SS, Mohanty S, Nayak SK. Composition analysis and characterization of waste polyvinyl chloride (PVC) recovered from data cables. Waste Manag. 2017;60:100–11. https://doi.org/10.1016/j.wasman.2016.08.033.
Goal. Department of Economic and Social Affairs Sustainable Development. https://sdgs.un.org/goals. 2023.
Ouyang J, Gu W, Zheng C, Yang H, Zhang X, Jin Y, et al. Polyethyleneimine (PEI) loaded MgO-SiO2 nanofibers from sepiolite minerals for reusable CO2 capture/release applications. Appl Clay Sci. 2018;152:267–75. https://doi.org/10.1016/j.clay.2017.11.023.
Dutcher B, Fan M, Russell A. Amine-based CO2 capture technology development from the beginning of 2013—a review. ACS Appl Mater Interfaces. 2015;7(4):2137–48. https://doi.org/10.1021/am507465f.
Ackley M. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003;61(1–3):25–42. https://doi.org/10.1016/s1387-1811(03)00353-6.
Beers A, Nijhuis T, Kapteijn F, Moulijn J. Zeolite coated structures for the acylation of aromatics. Microporous Mesoporous Mater. 2001;48:279–84. https://doi.org/10.1016/S1387-1811(01)00368-7.
Hollander M, Wissink M, Makkee M, Moulijn J. Gasoline conversion: reactivity towards cracking with equilibrated FCC and ZSM-5 catalysts. Appl Catal A. 2002;223:85–102. https://doi.org/10.1016/S0926-860X(01)00745-1.
Collignon F, Poncelet G. Comparative vapor phase synthesis of ETBE from ethanol and isobutene over different acid zeolites. J Catal. 2001;202(1):68–77. https://doi.org/10.1006/jcat.2001.3280.
Perez-Ramirez J, Kapteijn F, Mul G, Moulijn J. NO-assisted N2O decomposition over Fe-based catalysts: effects of gas-phase composition and catalyst constitution. J Catal. 2002;208(1):211–23. https://doi.org/10.1006/jcat.2002.3559.
Choi S, Drese J, Jones C. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. Chemsuschem. 2009;2(9):796–854. https://doi.org/10.1002/cssc.200900036.
Corma A, Fornes V, Navarro M, Perezpariente J. Acidity and stability of MCM-41 crystalline aluminosilicates. J Catal. 1994;148:569–74. https://doi.org/10.1006/jcat.1994.1243.
Ogura M, Shinomiya S, Tateno J, Nara Y, Nomura M, Kikuchi E, et al. Alkali-treatment technique—New method for modification of structural and acid-catalytic properties of ZSM-5 zeolites. Appl Catal A Gen. 2001;219(1–2):33–43. https://doi.org/10.1016/s0926-860x(01)00645-7.
Perez-Ramirez J, Kapteijn F, Groen J, Domenech A, Mul G, Moulijn J. Steam-activated FeMFI zeolites evolution of iron species and activity in direct N2O decomposition. J Catal. 2003;214(1):33–45. https://doi.org/10.1016/s0021-9517(02)00021-0.
Corma A, Martinez A, Martinez-Soria V. Catalytic performance of the new delaminated ITQ-2 zeolite for mild hydrocracking and aromatic hydrogenation processes. J Catal. 2001;200(2):259–69. https://doi.org/10.1006/jcat.2001.3219.
Li L, King D, Nie Z, Li X, Howard C. MgAl2O4 spinel-stabilized calcium oxide absorbents with improved durability for high-temperature CO2 capture. Energy Fuels. 2010;24(6):3698–703. https://doi.org/10.1021/ef100245q.
Daoudi M, Walters J. The reaction of HCl gas with calcined commercial limestone particles: the effect of particle size. Chem Eng J. 1991;47:11–6. https://doi.org/10.1016/0300-9467(91)85002-D.
Sun P, Grace J, Lim C, Anthony E. Determination of intrinsic rate constants of the CaO–CO2 reaction. Chem Eng Sci. 2008;63(1):47–56. https://doi.org/10.1016/j.ces.2007.08.055.
Daoudi M, Walters J. A thermogravimetric study of the reaction of hydrogen chloride gas with calcined limestone: determination of kinetic parameters. Chem Eng J. 1991;47:1–9. https://doi.org/10.1016/0300-9467(91)85001-C.
Karayıldırım T, Yanık J, Yüksel M, Sağlam M, Haussmann M. Degradation of PVC containing mixtures in the presence of HCl fixators. J Polym Environ. 2005;13(4):365–74. https://doi.org/10.1007/s10924-005-5531-2.
Lu J, Borjigin S, Kumagai S, Kameda T, Saito Y, Yoshioka T. Practical dechlorination of polyvinyl chloride wastes in NaOH/ethylene glycol using an up-scale ball mill reactor and validation by discrete element method simulations. Waste Manage. 2019;99:31–41. https://doi.org/10.1016/j.wasman.2019.08.034.
Lin Y. Production of valuable hydrocarbons by catalytic degradation of a mixture of post-consumer plastic waste in a fluidized-bed reactor. Polym Degrad Stab. 2009;94(11):1924–31. https://doi.org/10.1016/j.polymdegradstab.2009.08.004.
Nishibata H, Uddin M, Kato Y. Simultaneous degradation and dechlorination of poly (vinyl chloride) by a combination of superheated steam and CaO catalyst/adsorbent. Polym Degrad Stabil. 2020. https://doi.org/10.1016/j.polymdegradstab.2020.109225.
Sharma R, Segato T, Delplancke M, Terryn H, Baron G, Denayer J, et al. Hydrogen chloride removal from hydrogen gas by adsorption on hydrated ion-exchanged zeolites. Chem Eng J. 2020. https://doi.org/10.1016/j.cej.2019.122512.
Guo Y, Zhao C, Li C. Thermogravimetric analysis of carbonation behaviors of several potassium-based sorbents in low concentration CO2. J Therm Anal Calorim. 2014;119(1):441–51. https://doi.org/10.1007/s10973-014-4207-3.
Oliveira T, Santos M, Medeiros-Batista A, Morais-Araújo A, Conceição M, Fernandes V, et al. CaO–TiO2 bimetallic mixed oxide applied to the production of biodiesel from cotton oil (Gossypium hisutum): monitoring of the procedure by TGA. J Therm Anal Calorim. 2021;146(6):2667–79. https://doi.org/10.1007/s10973-021-10624-6.
Wajima T, Kuzawa K, Ishimoto H, Tamada O, Nishiyama T. The synthesis of zeolite-P, Linde Type A, and hydroxysodalite zeolites from paper sludge ash at low temperature (80 °C): Optimal ash-leaching condition for zeolite synthesis. Am Miner. 2004;89:1694–700. https://doi.org/10.2138/am-2004-11-1215.
Liu H, Wei G, Zhang R. Effect of water washing pre-treatment on the properties of glass-ceramics from incinerator fly ash using electronic arc furnace. J Wuhan Univ Technol Mater Sci Edn. 2013;28(1):62–8. https://doi.org/10.1007/s11595-013-0641-5.
Oliveira A, Kaviany M. Nonequilibrium in the transport of heat and reactants in combustion in porous media. Prog Energy Combust Sci. 2001;27:523–45. https://doi.org/10.1016/S0360-1285(00)00030-7.
Sun Y, Yuan B, Shang S, Zhang H, Shi Y, Yu B, et al. Surface modification of ammonium polyphosphate by supramolecular assembly for enhancing fire safety properties of polypropylene. Compos Part B Eng. 2020. https://doi.org/10.1016/j.compositesb.2019.107588.
Kongnoo A, Tontisirin S, Worathanakul P, Phalakornkule C. Surface characteristics and CO2 adsorption capacities of acid-activated zeolite 13X prepared from palm oil mill fly ash. Fuel. 2017;193:385–94. https://doi.org/10.1016/j.fuel.2016.12.087.
Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC) under Grant No. 52376123 and No. 52106146. The authors are indebted to Dr. Jiaqing Zhang and Dr. Yi Guo from State Grid Anhui Electric Power Research Institute for providing the cable materials.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by Yanyan Zou and Dennis Pau; methodology was done by Yanyan Zou and Yaoqiang Li; formal analysis and investigation were done by Yanyan Zou and Dennis Pau; writing—original draft preparation were done by Yanyan Zou; writing—review and editing were done by Dennis Pau and Kaiyuan Li; funding acquisition was done by Linlin Yi and Kaiyuan Li; resources were done by Linlin Yi and Kaiyuan Li; supervision was done by Linlin Yi and Kaiyuan Li.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, Y., Pau, D., Yi, L. et al. Optimizing in-situ capture of Cl and C through HCl acidification and crystal formation in PVC-based cable pyrolysis. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13216-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13216-2