Abstract
In this study, five different fuel blends were prepared by mixing biodiesel obtained from olive oil wastes using transesterification method, waste transformer oil, and Euro diesel in different ratios. The important physicochemical properties of the prepared fuel blends and produced biodiesel were determined by gas chromatography, Fourier transform infrared spectroscopy, and thermogravimetric analysis/differential scanning calorimetry analyses, and their characterizations were carried out. Then, the effects of the prepared fuel blends on engine performance and emission characteristics were investigated in a compression ignition engine. The experiments were performed with five different fuel blends (TD30, TD30B10, TD30B20, TD30B30, and D100) at 1000, 1500, 2000, and 2500 rpm. At all speeds, each fuel blend produced an average torque value that was highest for D100 fuel and lowest for TD30 fuel The average BP value produced by each fuel at all engine speeds was highest in D100 fuel and lowest in TD30 fuel. The results of the experiments showed that there was a 23.98% decrease in the average NOx emissions of TD30 fuel blend compared to the average NOx emissions of D100 fuel at all engine speeds. It was observed that all important fuel properties such as density, kinematic viscosity, and pour and cloud points of all fuel blends met the fuel standards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world meets the majority of its energy needs with fossil fuels. As the world's population continues to increase every year, the use of fossil fuels also increases. As the use of fossil fuel sources continues to increase, it is predicted that the number of reserves found will decrease within a century. The limited and highly probable exhaustion of fossil fuel reserves can make energy supply an economic and political issue for countries. The decrease in fossil fuel reserves, the increasing damage to nature and the environment, the constant rise in oil prices, and the harmful emissions released by burning oil-based fuels leading to air pollution have caused countries to turn to alternative energy sources.
In developed countries, the use of biological fuels such as biogas, synthetic fuels, and vegetable oils has gained importance. It has been proven that biological fuels have similar properties to diesel fuel, which is derived from petroleum, and can be used directly as a substitute for diesel fuel or with some improvements [1].
Biodiesel, a derivative of renewable energy sources, has many advantages over other energy sources in terms of being environmentally friendly, containing oxygen, reducing particulate and carbon monoxide emissions, allowing vegetable waste oil to be used as fuel, and having similar properties to diesel fuel. Biological additives have attracted attention due to their advantages in reducing exhaust emissions by being biodegradable and non-toxic in nature [2]. In addition to these advantages, the ability to be blended with diesel fuel at certain ratios also increases the use of biodiesel [3].
The main raw materials used in the production of biodiesel are animal and vegetable oils. Rudolph DIESEL demonstrated the usability of vegetable oils as fuel by running a diesel engine with peanut oil for the first time in the 1900s. Studies are being conducted to utilize various wastes as fuel, such as waste vegetable oils [4,5,6,7,8,9,10,11,12,13], waste plastics [14], waste tires [15], plastic oil blends [16,17,18,19], used engine oils [20], and waste transformer oils [21,22,23,24]. Biodiesel production from waste vegetable oils is carried out by the transesterification method. The biodiesel produced can be used as fuel in compression ignition engines without any modifications. Unlike waste vegetable oils, fuel is obtained from waste engine oils and used tires using the pyrolysis method [25].
Belkhode et al. [24] investigated the use of waste transformer oil, which has been exposed to long-term thermal stress in transformers, as an alternative fuel type by subjecting it to atmospheric pressure distillation in their study. It was observed that waste transformer oil yielded better results at lower mixture ratios [24].
Basha et al. [26] review of studies investigated the production of biodiesel from different sources and its effects on engine performance. According to their findings, using vegetable oils as fuel in compression ignition engines resulted in good performance in engine tests, but led to lubricating oil contamination and excessive carbon accumulation in the engine when it ran for long periods. To address this issue, they recommended mixing diesel fuel with biodiesel in certain proportions. Biodiesel produced from vegetable oils exhibited properties similar to diesel fuel, with a higher ignition pressure, higher ignition temperature, and shorter ignition delay times when mixed with diesel fuel. They also observed that the engine power was equivalent to that of diesel fuel and that more effective alternative fuels could be obtained through the use of certain catalysts and enzymes in the production of biodiesel [26].
Balat and Balat [22] produced a biodiesel with properties similar to petroleum-derived diesel from rapeseed, soybean, and waste cooking oils. However, the viscosities of these oils are very high, which can damage engine injection systems. To address this problem, transesterification is used as a conversion process to produce biodiesel. Other methods such as pyrolysis, dilution, and microemulsion can also be applied. Transesterification is the most commonly used method. Methyl esters produced by transesterification of vegetable oils have lower viscosity, a higher cetane number, and an improved calorific value compared to pure vegetable oils. Due to these properties, engine tests resulted in shorter ignition delays, longer burning times, and lower particulate emissions [27].
Prasanna et al. [28] conducted study on the use of waste transformer oil as a fuel in a compression ignition engine in order to address environmental issues associated with its disposal. They utilized catalytic cracking to break down the waste transformer oil, resulting in waste oil and catalyst residue products. The use of activated volatile ash as a catalyst showed that the catalytic cracking process could occur at lower reaction temperatures, between 350 and 400 °C. The waste oil produced through catalytic cracking met ASTM standards. The engine tests showed that CCWTO 50 (50% diesel, 50% catalytically cracked waste transformer oil) had a 7.4% increase in brake thermal efficiency compared to diesel fuel and exhibited improved combustion characteristics. However, NOx emissions increased, while HC, CO, and smoke emissions decreased [28].
Ajay et al. [29] investigated waste transformer oil as an alternative fuel. They first applied transesterification to the waste transformer oil and refined it. The refined waste transformer oil (BD) was then blended with diesel fuel at certain ratios for the engine experiments. These blends were BD100, B10, B20, B30, B40, and D100. After conducting engine tests on a single-cylinder compression ignition engine with the prepared fuel blends, they concluded that the alternative fuel blend showing the best engine performance, emission, and combustion characteristics was B30 [29].
Sethuraman et al. [30] conducted a study on the emission characteristics and engine performance of waste cooking oil and waste transformer oil methyl esters mixed with diesel in a single-cylinder water-cooled direct injection compression ignition engine. In their study, a catalyst was produced from coconut shells and used as a catalyst to produce biodiesel from both waste transformer oil and waste cooking oil through transesterification with methanol. Biodiesel blends (B20 + D80) and (B40 + D60) were prepared from both waste transformer oil and waste cooking oil. According to the experimental results, the B20 blend increased the BTE and BSFC compared to diesel. In terms of emissions, CO emissions decreased, while HC and NOx emissions increased [30].
Singh and Kumar [31] conducted a study in which they compared the engine performance and emission characteristics of butanol–biodiesel and butanol–biodiesel–diesel fuel blends in diesel engines. The study found that the triple butanol–biodiesel–diesel fuel blend resulted in a significant decrease in engine performance compared to butanol–biodiesel and biodiesel–diesel fuels, but only a small decrease compared to diesel fuel. The combustion characteristics of the triple butanol–biodiesel–diesel fuel blend were similar to those of the dual biodiesel–diesel fuel blend. The study also found that the triple butanol–biodiesel–diesel fuel blend provided the best reduction in emissions. As a result, the study concluded that butanol has the potential to help reduce engine performance and emissions. They observed that a butanol–diesel fuel blend containing 20% butanol provided better engine performance results than diesel fuel [31].
Öztürk [32] investigated the engine performance, emission, injection, and combustion characteristics of blends of diesel fuel with biodiesel produced from canola oil and hazelnut soapstock in a diesel engine. In the study, biodiesel produced from canola oil and hazelnut soapstock was mixed with diesel fuel to improve biodiesel properties and reduce fuel costs. Engine tests were performed with a single-cylinder direct injection diesel engine using fuel blends containing 5% and 10% biodiesel and diesel fuel samples. The experimental results showed that the addition of biodiesel to diesel fuel reduced injection and ignition delays and maximum heat release rates, while increasing injection and combustion durations. They proved that the fuel blend that exhibited the best combustion characteristics contained 5% biodiesel. They also observed that the fuel blend containing 10% biodiesel disrupted combustion due to its high density and viscosity [32].
Dehghan et al. [33] conducted a study on the microwave-assisted transesterification of olive oil for biodiesel production. The variables of the transesterification reaction affected the yields, physicochemical properties, and purity of methyl esters. The variables of the reaction were reaction time (3–15 min), methanol–oil molar ratio (3/15), microwave power level (100–900 W), and catalyst concentration (0.4–2.0%). The results showed that the yield and purity of methyl esters in biodiesel increased parallel to the increase in catalyst amount, reaction time, methanol-to-oil molar ratio, and power level. The microwave-assisted transesterification method can increase the yield of methyl esters more than the traditional magnetic stirrer transesterification method and reduce the reaction time and energy consumption. Therefore, the microwave-assisted transesterification method can be used as a fast method for biodiesel production from olive oil [33].
In the literature, it is seen that obtaining alternative fuels from recycled materials and vegetable waste is more important than researching new fuels as an alternative to diesel. In this study, alternative fuels were produced by blending biodiesel fuel obtained from olive oil wastes with the transesterification method in different ratios with used waste transformer oils at the end of their service lives. Firstly, the alternative fuels produced were characterized by various analyses such as GC, TG-DSC, FTIR, density, viscosity, cloud point, pour point, and acid value. Based on these analyses, it was concluded that all blends were similar to diesel fuel and could be used for diesel engines. Afterward, engine performance and exhaust emission tests were conducted on all fuel samples using a compression ignition (CI) engine. With this experimental study, the recycling of olive oil wastes and used transformer oils was achieved, and low-unit energy cost fuel production was realized. Additionally, an economically advantageous alternative to diesel fuel was produced. Thus, the aim was to minimize the damage to the environment by recycling waste products.
Objectives
This manuscript aims to present the findings of an experimental investigation into the feasibility and efficacy of utilizing olive oil wastes and waste transformer oils as alternative fuel sources in compression ignition engines. The specific objectives pursued in this study are as follows:
Exploration of Alternative Fuel Types: The primary objective is to assess the viability of incorporating olive oil wastes and waste transformer oils as alternative fuels for compression ignition engines, both within the context of Turkey and on a broader global scale.
Low-Unit Energy Cost Fuel Production: This study seeks to contribute to the discourse on sustainable energy practices by investigating methodologies for repurposing olive oil wastes and waste transformer oils to produce low-cost fuels. By doing so, the aim is to enable the recycling of these waste materials, thereby potentially mitigating environmental burdens while offering economically viable fuel options.
Characterization of Fuel Properties: A fundamental aspect of this research involves characterizing the thermochemical and physicochemical properties of biodiesel, waste transformer oil, and diesel blends. Through comprehensive analysis, the objective is to elucidate crucial parameters such as viscosity, calorific value, density, and chemical composition to assess the suitability of these blends for use in compression ignition engines.
Evaluation of Engine Performance and Emissions: The manuscript endeavors to provide insights into the performance and exhaust emissions of blends derived from olive oil wastes, waste transformer oils, and conventional diesel. By evaluating factors such as power output, fuel efficiency, combustion characteristics, and emission profiles, the aim is to ascertain the overall effectiveness and environmental impact of utilizing these alternative fuel blends.
By addressing these aims, the manuscript endeavors to contribute to the body of knowledge surrounding sustainable energy solutions, offering valuable insights into the potential utilization of waste-derived fuels in compression ignition engines. Ultimately, it is anticipated that the findings presented herein will inform future research endeavors and policy initiatives aimed at advancing sustainable energy practices and mitigating environmental challenges.
Materials and methods
In this study, biodiesel, waste transformer oil, and euro diesel (D100) fuel blends were prepared. First, the thermodynamic and physicochemical properties of these fuel blends were determined, and their characterization was carried out. Then, engine performance and exhaust emission tests were conducted. Detailed information is provided on the devices and methods used for all test and analysis procedures.
Production of biodiesel from olive oil wastes using the transesterification method
The procedure for biodiesel production involves the reaction of a vegetable oil with methanol, in the presence of a catalyst (NaOH), to produce fatty acid alkyl esters (biodiesel) and glycerol through a process called transesterification. The steps involved in the transesterification method are illustrated in Fig. 1 and were carried out in the Food Processing Laboratory of the Atatürk University Technical Sciences Vocational School. Sodium hydroxide (NaOH) was used as the catalyst and methanol (CH3OH) as the alcohol for the transesterification process. The chemical and physical properties of NaOH and methanol are shown in Table 1. Olive oil wastes were used as the vegetable waste oil in this method.
The first step of the transesterification method involved dissolving 10 g of sodium hydroxide (NaOH) catalyst in 333 g of methyl alcohol in a glass reactor equipped with a heating magnetic stirrer at 30–40 °C for 30 min until completely dissolved. This resulted in the formation of a CH3OH-NaOH solution (Fig. 1a). To avoid any loss, olive oil wastes and olive seed oil were added to a glass reactor equipped with a reflux condenser. The reactor was heated to 60 °C to promote the reaction. Then, the heated waste vegetable oils were slowly added to the two-necked glass reactor along with the catalyst/alcohol (CH3OH-NaOH) mixture while being stirred. The reaction was maintained at 60 °C for 5 h. The experimental setup for the reaction is shown in Fig. 1b.
After the completion of the reaction, the resulting products were separated using a separating funnel. After waiting for a certain time at room temperature, biodiesel was found to be on top due to its density, while glycerol settled at the bottom. The glycerol that settled at the bottom was drained. However, it was observed that a small amount of glycerol remained in the biodiesel. To remove the glycerol, the mixture was washed twice with warm (30–35 °C) water, and the glycerol that settled at the bottom was drained again. As a result of this process, pure biodiesel was obtained from the reaction vessel (Fig. 1c). After the separation of biodiesel and glycerol phases, the remaining methyl alcohol in biodiesel and glycerol was removed using the distillation method by taking advantage of the difference in boiling points (Fig. 1d). The resulting biodiesel (B100) is shown in Fig. 2.
Waste transformer oil
The waste transformer oil, which is one of the alternative fuel blends used in the experiment, was obtained from the TEIAS Erzurum Regional Directorate. The obtained waste transformer oil (T100) is shown in Fig. 2.
The obtained waste transformer oil was purified from water and sediment before being used in the experiment and made ready for use. Afterward, test sample blends were prepared by mixing the waste transformer oil with the produced biodiesel and diesel fuel in the ratios given in Table 2.
Preparation of biodiesel, waste transformer oil, and euro diesel fuel blends
In the experimental study, fuel blend samples were prepared according to the volume percentages given in Table 2. When preparing the fuel blends, all fuel types were passed through a filter.
During the experiment, fuel blend samples were prepared according to the volumetric percentages given in Table 2. All fuel types were passed through a filter during preparation of the fuel blends.
The fuel blend samples were prepared to include biodiesel proportions of 10%, 20%, and 30% of the total volume. The proportion of waste transformer oil and Euro diesel fuel in the fuel blends was, for example, 10% biodiesel by volume, while the remaining 90% of the fuel blend was composed of 30% waste transformer oil and 70% Euro diesel fuel, as in test sample 3. The overall volume percentage of Euro diesel fuel in the samples was 100%, 70%, 63%, 56%, and 49%, respectively.. As can be seen, by keeping the proportion of Euro diesel fuel high in the fuel blends, knock-free combustion formation was achieved, and engine tests were performed. Figure 4 shows the seven different fuels and fuel blends prepared from waste transformer oil, biodiesel, and Euro diesel fuel.
Characterization of biodiesel, euro diesel, waste transformer oil, and fuel blends
Biodiesel, diesel, waste transformer oil, and ternary fuel blends were tested for some physicochemical properties (density, kinematic viscosity, and pour and cloud points) according to ASTM D6751 and EN 14214 standards. Additionally, the fatty acid composition of biodiesel produced from olive oil wastes was determined. Details of the various analyses performed on the samples are provided in Table 3.
The fatty acid composition of the biodiesel was determined by gas chromatography (GC) at an initial oven temperature of 140 °C for 5 min. Then, the oven temperature was increased to 240 °C at a rate of 3 °C/min and held at this temperature for 17 min. FTIR analysis was performed in the range of 4000–400cm−1.
TG/DSC analysis was carried out in an air atmosphere at a heating rate of 10 °C/min in the range of 25–600 °C.
The acid values of the triple fuel blends containing biodiesel were determined by the titration method using a KOH–ethanol solution. Samples weighing 5–10 g were dissolved in diethyl ether in an Erlenmeyer flask. After adding 3–4 drops of phenolphthalein indicator, the solution was titrated with 0.1 N KOH (a solution containing 5.61 g KOH in 100 mL of ethanol) until a pink color was obtained. The amount of KOH solution used was recorded, and the acid value was calculated using formula 1.
Here, V is the amount of consumption during titration (mL) and m is the mass of the sample (g).
The physical and chemical properties of the waste transformer oil, Euro diesel fuel obtained from Erzurum Shell Petroleum, and the produced biodiesel were determined in the Atatürk University Department of Chemical Engineering laboratory.
Compression ignition test engine characteristics, engine performance, and emissions
The engine tests were conducted at the Engine Laboratory of the Department of Mechanical Engineering at Dicle University. The tests were performed on an Antor (4 LD 820) engine, which is a compression ignition, four-stroke, single-cylinder, air-cooled engine. The fuel samples were prepared and tested in this engine. During the tests, it was observed that the engine operated smoothly without approaching the knock limit. The tests were conducted at full load and at four different speeds (1000, 1500, 2000, and 2500 rpm).
Compression ignition engine characteristics and control unit
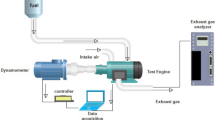
The experiment was carried out by performing engine performance and exhaust emission analyses using a compression ignition, four-stroke, single-cylinder, and air-cooled Antor (4 LD 820) brand engine. A water brake dynamometer was connected to the compression ignition engine to adjust the engine loads or speeds. An electronic control and measurement device were used to measure the engine speed (rev/min), torque (kgm), and brake power (kW). The electronic control panel used is shown in Fig. 3. The measurement accuracy of the engine speed and brake power of the electronic control unit is ± 5 rev/min and ± 0.5055, respectively.
Engine test rig and components [34]
After the prepared fuel blends were poured into the fuel tank, they were passed through a tube with a valve control and a 50 and 100 mL glass fuel burette with a control valve (Fig. 3). The combustion time of a 50 mL fuel sample in the compression ignition experiment engine was measured using a stopwatch. Before the engine tests of the experiment samples, the engine was run for 10 min using Euro diesel (D100) fuel to stabilize the engine. Figure 3 shows the compression ignition test engine, dynamometer, and schematic representation.
The engine performance and exhaust emission values were measured for each test sample. The experiments were first run at 3000 rpm to allow the engine to reach a stable state, and then the load applied to the test engine was increased using the water brake dynamometer. As a result, the engine speed was reduced to 2500, 2000, 1500, and 1000 rpm, and the test data were measured. The technical specifications of the compression ignition test engine are shown in Table 4.
Exhaust analyzer
For measuring the exhaust emissions (CO, NOx, and exhaust gas temperature) released from the compression ignition test engine, a Testo 350 exhaust gas analyzer was used. The Testo 350 device consists of three parts, as seen in Fig. 3: the body, the control unit, and the probe. With this device, CO, NOx, and SO2 emissions can be measured with 10% accuracy, NO2 emissions with 5% accuracy, and exhaust gas temperature with ± 1 °C accuracy. The technical specifications of the device's exhaust gas measurement are shown in Table 5.
When the compression ignition test engine reached its operating temperature for each test sample, the exhaust emission measurement probe was placed in the hole of the exhaust pipe. The emission values were measured with the probe, and this measurement was repeated twice. The measurement results were recorded in the measurement device, and after the experimental procedures for all samples were completed, the measurement values taken at 2500, 2000, 1500, and 1000 rpm were printed from the device's printer.
Compression ignition engine performance and calculations
The engine performance and characteristics were examined for each fuel sample in a compression ignition test engine at four different speeds (2500, 2000, 1500, and 1000 rpm). Performance parameters such as torque (T), brake power (BP), and brake specific fuel consumption (BSFC) were analyzed for each fuel sample. Torque is the rotational force generated by the crankshaft of an engine. The more torque an engine produces, the greater its ability to do work. The torque value can be calculated using formula 2.
T is the torque (Nm), F is the force (N), and d indicates the distance in meters (m) from the center of the rotor.
The break power provided by the compression ignition engine and absorbed by the water brake dynamometer is calculated by the equation in formula 3.
where BP is the break power (kW), ω represents the angular velocity (rad s−1)
The ratio of the unit value of fuel consumed by the engine at the time of operation to the resulting engine power in g k−1 W−1 h−1 gives the specific fuel consumption. Brake specific fuel consumption (BSFC) is calculated by formula 4.
BSFC is the brake specific fuel consumption (g k−1 W−1 h−1), \(\dot{{m}_{{\text{f}}}}\) is the fuel mass flow (kg h−1).
Results and Discussion
Chemical and physical properties analysis of prepared fuel blends
In this section, gas chromatography (GC), some important fuel properties of the samples, and the results of FTIR and TG/DSC analyses are examined.
Gas chromatography analysis
A biodiesel sample produced from olive oil wastes was analyzed for fatty acid methyl ester composition using gas chromatography (GC). Figure 4 shows the gas chromatogram of the biodiesel sample, and Table 6 lists the major compounds present in the sample. The biodiesel produced from olive oil wastes consists of both saturated (palmitic acid C16:0, stearic acid C18:0) and unsaturated (oleic acid C18:1, linoleic acid C18:2, palmitoleic acid) fatty acid methyl esters (FAMEs). The predominance of unsaturated fatty acids in the biodiesel sample is due to its vegetable oil origin. The most abundant unsaturated fatty acid is oleic acid (approximately 70%). Olive oil is a rich source of oleic acid [35]. Therefore, it is expected that the percentage of oleic acid will be high in biodiesel derived from olive oil. As it is known, the fatty acid profile of biodiesel with high levels of fatty acids affects important fuel properties such as density, viscosity, pour point, and cloud point [36, 37].
Analysis of fuel properties of samples
Some physicochemical properties of biodiesel, diesel, waste transformer oil, and fuel blends were examined, and the results are given in Table 7.
Fuel density is one of the important fuel properties that affects engine performance. Fuel injection systems measure fuel on a volume basis, so a higher or lower fuel mass is injected depending on the density [38]. When Table 7 is examined, it can be seen that the densities of all samples except B100 vary in the range of 830–880 and all values comply with ASTM standards. The density of B100, which has the highest density, is slightly above both standard ranges with a value of 912.4. This value is expected for biodiesel produced from olive oil [39].
Kinematic viscosity is an important fuel property that indicates the flowability of the fuel. High viscosity will reduce the fluidity of the fuel and negatively affect the operation of fuel injection equipment and spray atomization [34]. As seen in Table 7, the kinematic viscosity values of all samples except T100 and B100 vary between 3 and 5.8 mm2 s−1, and meet the standards. The kinematic viscosity values of T100 and B100 are 10.6 mm2 s−1 and 34.1 mm2 s−1, respectively. Although these values are outside the standard range, they are also quite high. It is known that waste transformer oils are mixed with diesel fuel in different ratios for use due to their high viscosity values [40]. The high viscosity of the biodiesel sample derived from olive oil is also an expected result. Similar results were also obtained in the study by Ayadi et al. [41].
Cloud point and pour point are the most important cold flow properties of fuels. The cloud point is the temperature at which the first solid crystals begin to form in the fuel during cooling. At this temperature, a cloudy suspension begins to form in the fuel that has the potential to clog filters. Pour point, on the other hand, is the temperature at which there is sufficient wax in the solution for the fuel to gel as cooling continues. At this temperature, the fuel becomes too thick to be pumped [42]. As seen in Table 7, the cloud point (+ 4 °C) and pour point (− 8 °C) of B100, which has the highest kinematic viscosity, are not very low temperatures. The pour points of D100, T100, and TD30 samples are − 35 °C, ≤ − 40 °C, and ≤ − 40 °C, respectively, which are quite low temperatures. However, it is observed that as the biodiesel content increases in the triple fuel blends (TD30B10, TD30B20, TD30B30), the cloud point and pour point increase. This is due to the poor cold flow properties of B100, which also negatively affect the triple fuel blends mixed with it. Since no standard range is specified for pour point and cloud point, all samples are considered acceptable.
High acid value increases corrosion and is undesirable in fuels. Biodiesel contains fatty acids due to its structure [43]. In this study, it is observed that the acid value of B100 is very high, at 19.51 mgKOH g−1. In addition, it is observed that the acid values of the samples mixed with biodiesel (TD30B10, TD30B20, TD30B30) increase as the biodiesel ratio in the blends increases, and the acid values are much higher than the standard ranges (Table 7). This is again due to the very high acid value of B100.
FTIR analysis
FTIR plots provide a fingerprint-like view of the molecule, indicating the presence of various functional groups. The infrared spectra of biodiesel (B100), diesel (D100), waste transformer oil (T100), and their blends are shown in Fig. 5 in this study.
The presence of various peaks and dips throughout the spectrum denotes several functional groups. Accordingly, it was observed that all samples gave peaks at 2921 cm−1, 2852 cm−1, and 1463 cm−1. Peaks around 3000–2900 cm−1 are attributed to due to C–H stretching (associated with alkanes or aromatics). Strong peaks showing the C=O stretching vibration of ester carbonyl groups at 1743 cm−1 were observed in biodiesel (B100) and its triple blends (TD30B10, TD30B20, and TD30B10).The absorption band intervals and the functional groups attributed to them are shown in Table 8 for all samples [42].
TG/DSC analysis
The TG-DSC analysis of TD30B10 sample, which exhibited the best combustion performance, was performed, and the resulting TGA curve is shown in Fig. 6. The experiments were conducted in an air environment at a heating rate of 10 °C/min and in the temperature range of 25–600 °C. It is observed that the sample has completely reacted after this period. A weak exothermic peak is seen in the DSC curve for this reaction. The reaction starting temperature for the TD30B10 sample is around 55 °C, and the reaction ending temperature is around 280 °C.
The TGA curve shows a significant decrease in mass starting at around 100 °C and ending around 500 °C. This suggests that the sample undergoes a major decomposition process within this temperature range, losing about 99% of its initial mass. In particular, the material decomposed significantly around 220–230 °C, losing most of its mass in the process. On the other hand, the DSC curve shows a broad peak around 220–230 °C. This peak likely corresponds to the same decomposition process observed in the TGA curve.
Engine performance tests
Five different fuel blends were prepared and their engine performance tests were conducted on a compressed spark ignition, direct injection, four-stroke, single-cylinder, air-cooled engine. The ratios and amounts of the prepared fuel blends are shown in Table 2. The characteristics and schematic image of the compressed spark ignition engine are shown in Table 4 and Fig. 3, respectively. With these engine tests, the performance properties of 5 different fuel blends (D100, TD30, TD30B10, TD30B20, and TD30B30) such as torque (T), brake power (BP), and brake specific fuel consumption (BSFC) were examined. During the engine tests of the prepared fuel blends, the compression ignition engine was first operated for a certain period of time with Euro diesel (D100) until the engine stabilized. First, all performance and emission measurements were taken for Euro diesel (D100) at 2500 rpm. These processes were then repeated for Euro diesel (D100) fuel sample at 2000 rpm, 1500 rpm, and 1000 rpm, respectively. After the measurements for the Euro diesel (D100) fuel sample, the same measurements were taken for the other fuel samples (TD30, TD30B10, TD30B20, and TD30B30) in Table 2, while each fuel sample was operated with Euro diesel (D100) at idle for 30 min to clean the previous fuel from the engine fuel line and cool the engine before each engine test. In this way, the measurements were taken with a lower error rate.
In the engine performance experiments conducted on the compression ignition engine, a water brake dynamometer was used to measure load and torque. After the engine was started and heated, the engine speed was fixed at 3000 rpm, and then, with the help of the water brake, the engine load was increased, and steady-state conditions were established for each of the following speeds: 2500 rpm, 2000 rpm, 1500 rpm, and 1000 rpm. After reaching steady-state conditions, measurements were made for each speed for engine performance and emission parameters. Subsequently, the measured values were plotted and each engine performance parameter was interpreted separately based on the calculations.
Torque (T)
Torque values produced at four different engine speeds of five different fuel blends made in a compression ignition test engine are shown in the graph in Fig. 7.
The graph in Fig. 7 shows that Euro diesel (D100) fuel had the highest torque values compared to other fuel blends, except for the TD30B30 fuel blend at 1000 rpm, at all engine speeds tested. For all fuel blends, the torque value decreases as the engine speed increases.
The highest torque values of all fuel blends were obtained at the low engine speed of 1000 rpm. The results were measured as 36.53 Nm for D100 fuel, 33.40 Nm for TD30 fuel blend, 35.41 Nm for TD30B10, 35.17 Nm for TD30B20 fuel blend, and 36.74Nm for TD30B30 fuel blend. According to the measurements, it was found that the torque values were quite close to each other. According to the fuel blends, it was determined that the torque values were higher for the fuel blends TD30B10, TD30B20, and TD30B30, which were created by adding biodiesel fuel to the TD30 fuel blend, compared to the TD30 fuel blend. It was observed that the torque values increased by adding biodiesel to the fuel blends.
When the average torque values of fuel blends are compared with the average torque values of Euro diesel (D100) at all engine speeds, it was determined that there was a decrease of 11.27% in TD30 fuel blend, 6.48% in TD30B10 fuel blend, 8.23% in TD30B20 fuel blend, and 9.67% in TD30B30 fuel blend.
The reason for this is that diesel, waste transfer oil, and waste seed oil methyl ester mixtures have higher density and viscosity and lower calorific value and pour point compared to diesel fuel. Additionally, as engine speed increases, mechanical friction increases and brake power and torque decrease rapidly [44].
Brake power (BP)
The five different fuel blends tested in the compression ignition test engine were measured in terms of produced brake power at four different engine speeds. Brake power, also known as useful power of the engine, was measured by the dynamometer through the force it measures, and the values were recorded electronically and plotted on the graph in Fig. 8.
When examining the graph in Fig. 8, it can be seen that the Euro diesel (D100) fuel has achieved the highest brake power values compared to other fuel blends in all experiments conducted at all engine speeds except for the TD30B30 fuel blend at 1000 rpm. For all fuel blends, the brake power increased as the engine speed increased.
When examining the fuel blends, it has been determined that the addition of biodiesel fuel to TD30 fuel blend to create TD30B10, TD30B20, and TD30B30 fuel blends results in higher brake power values compared to TD30 fuel blend. The average brake power values of fuel blends at all engine speeds were compared with the average brake power value of Euro diesel (D100) fuel. It was determined that there was a decrease of 13.59% in the TD30 fuel blend, a decrease of 8.93% in the TD30B10 fuel blend, a decrease of 11.30% in the TD30B20 fuel blend, and a decrease of 13.37% in the TD30B30 fuel blend. It is observed that the uneven combustion characteristics of fuel blends particularly caused a significant decrease in engine power at 2500 rpm.
The maximum BP for fuel blends is lower than for pure diesel fuel. This is due to the combined effects of high relative fuel density, viscosity and low heating value. BP and braking torque decrease more rapidly as engine speed increases due to the increased value of high mechanical frictions. Poor spray properties of fuel mixtures reduce BP by affecting the homogeneity of the air/fuel mixture [44].
Brake specific fuel consumption (BSFC)
The brake specific fuel consumption (BSFC) represents the fuel consumption per unit power, and as a result, the amount of fuel required for all blends to produce the same power output has been determined. The BSFC values for five different fuel blends tested on a compression ignition engine at four different engine speeds are shown in Fig. 9.
Upon examining the graph in Fig. 9, it can be seen that Euro diesel (D100) fuel has the lowest specific fuel consumption value compared to other fuel blends at all engine speeds in the experiments conducted. For all fuel blends, except for the 1500 rpm engine speed, specific fuel consumption values have increased as the engine speeds increased.
Looking at the fuel blends, it has been determined that the specific fuel consumption values of TD30B10, TD30B20, and TD30B30 fuel blends, which were created by adding biodiesel fuel to the TD30 fuel blend, are generally lower than TD30 fuel at all engine speeds. When the average specific fuel consumption values of fuel blends were compared with the average specific fuel consumption value of Euro diesel (D100) at all engine speeds, it was determined that there was an increase of 25.4% in the TD30 fuel blend, 18% in the TD30B10 fuel blend, 20.1% in the TD30B20 fuel blend, and 24.8% in the TD30B30 fuel blend.
Compared to pure diesel fuel, fuel blends have higher density and lower heating values, which are the main reasons for the decrease in BP and torque. Hence, difference in fuel densities affects BSFC, BP, and torque. It will be necessary to obtain the same power when spraying when all other conditions remain the same. The delicate distribution of fuel/air “packets” within the sprays can be higher for all fuel blends than pure diesel fuel, increasing the fuel required per cycle [44].
Exhaust emissions
During the engine test of the five different fuel blends prepared, emission measurements were taken using the Testo 350 exhaust emission measurement device shown in Fig. 3, and the measurements were recorded in the device. Engine performance values were taken for each fuel blend at four different speeds under the heading of engine performance analysis, and after the engine performance and emission measurements of all fuel samples were completed, the exhaust emission measurement values of all fuel samples were printed from the printer of the complex device. Afterward, the measurement values obtained were plotted and separately interpreted for each emission under this heading.
CO emissions
CO (carbon monoxide) is odorless and colorless, and it hinders the transportation of oxygen in the bloodstream when inhaled. This leads to severe functional disorders in the heart and brain. The reason why the combustion products should not contain CO molecules is due to the insufficient oxygen in the combustion chamber [45]. Figure 10 shows the variations in CO emissions for all fuel blends at 1000, 1500, 2000, and 2500 rpm engine speeds.
When examining the graph in Fig. 10, it can be seen that as the engine speed increases, the CO emission value tends to decrease for all fuel blends tested. When compared with the CO emission values of Euro diesel (D100) fuel, it is observed that the CO emission value of TD30 fuel blend is high at 1000 and 1500 rpm engine speeds, while it is lower at other speeds. The reason for the high CO emission values for all fuel blends at 1000 rpm engine speeds can be explained by insufficient air and combustion at high temperatures [46]. As a result of increased air movements at high engine speeds, combustion occurred more efficiently, and the CO emission value decreased. When the average CO emissions measured at all engine speeds for each fuel blend were compared with Euro diesel (D100) fuel, it was determined that there was a 4.61% increase in TD30 fuel blend, an 11.8% decrease in TD30B10 fuel blend, a 23.87% decrease in TD30B20 fuel blend, and a 37.78% decrease in TD30B30 fuel blend. As the biodiesel ratio in the blends increased and the percentage of waste transformer oil decreased, CO emission levels gradually decreased at 1000 and 1500 rpm engine speeds. At 2000 and 2500 rpm engine speeds, the rate of decrease in CO emission levels remained approximately the same. As a result of the investigation, it was observed that TD30B10, TD30B20, and TD30B30 fuel blends obtained very good results due to the oxygen present in the structure of biodiesel, compared to the average CO emission values measured for Euro diesel (D100) fuel at all engine speeds. In Fig. 10, while the engine speed was reduced from 2500 to 1000 rpm, the CO rate increased for all fuel samples. It is seen that as the biodiesel ratio in the TD30 sample increases, the CO ratio decreases. It is thought that this decrease depends on the amount of oxygen in biodiesel. This situation is seen at all engine speeds.
Higher CO emissions for fuel mixtures at low speeds compared to pure diesel fuel can be attributed to local rich zones and lean mixture formation in some parts of the combustion chamber. This is likely a result of poor spray atomization and non-uniform mixing formation of ternary fuel mixtures [47, 48].
NOx emissions
The graph in Fig. 11 shows the changes in NOx emissions for all fuel blends at 1000, 1500, 2000, and 2500 rpm engine speeds. The main reason for the release of NOx during combustion is the excessively high oxygen content in the air taken into the combustion chamber during combustion at high temperatures [45]. When the combustion process takes place at a temperature above 1800 K, the oxygen and nitrogen in the air combine chemically to form the NOx molecule [49]. This mechanism of NOx formation is also referred to as the Zeldovich mechanism [46]. When diesel and gasoline fueled engines are compared, the amount of NOx emissions released is 123.71 and 18.42 kg per ton, respectively [50].
When Fig. 11 is examined, it is seen that the NOx emission values of triple fuel blends are lower than the NOx values of Euro diesel (D100) fuel at almost all engine speeds.
In the engine tests, when the NOx emission values of the TD30B10 and TD30B30 fuel blends were compared with the Euro diesel fuel NOx emission values at 1000 rpm engine speed, an increase of 1.3% and 13% was observed, respectively. The reason for this is the occurrence of bad combustion with excessive load and insufficient air at low engine speeds. At 1000 rpm engine speed, the highest NOx formation was in the TD30B30 fuel blend, and the lowest NOx formation was in the TD30 fuel blend. The highest NOx formation was observed in Euro diesel (D100) fuel at all other engine speeds. With the increase in the biodiesel ratio in the blends and the decrease in the waste transformer oil percentage, the NOx emission levels increased slightly at the 1000 and 1500 rpm engine speeds, while the NOx emission level gradually decreased at the 2000 and 2500 rpm engine speeds. At 2000 and 2500 rpm engine speeds, the temperature decreased under low load condition and with increasing air movements. As a result, a high reduction in NOx emission levels was achieved.
When the average values of NOx emissions at all engine speeds are compared with the average values of Euro diesel (D100) fuel, it is observed that there is a 23.98% decrease in TD30 fuel blend, 14.92% decrease in TD30B10 fuel blend, and 18.78% decrease in TD30B20 fuel blend and TD30B30 fuel blend. It was determined that there was a decrease of 14.03%. Normally, the amount of water in biodiesel is expected to increase NOx formation by increasing the pressure and temperature after combustion.
Higher density fuels generally contain more oxygen, which can result in higher combustion temperatures and more NOx formation. High-density fuels can burn more quickly and completely, which can increase NOx emissions. Additionally, higher viscosity in fuels can often reduce combustion efficiency because atomization and mixing of the fuel may become difficult. In this case, there may be more partial combustion or non-combustion reactions during combustion, which may increase NOx formation. For these reasons, it is thought that the amount of NOx increases in blends where the biodiesel ratio increases due to its high density and kinematic viscosity, especially at low speeds close to idle speed (1000–1500 rpm).
NOx formation depends on the amount of oxygen in the environment, combustion chamber temperature and exposure time [51]. When Fig. 11 is examined, it is seen that NOx formation increases depending on the biodiesel ratio at 1000 rpm when the engine is at maximum load. It is thought that this situation is due to the oxygen present in the structure of biodiesel and the fact that the fuel–air mixture remains in the combustion chamber for a longer time. This situation is supported by the literature [48].
The low calorific value of the fuel, combined with the high latent heat of vaporization, leads to lower cylinder flame temperatures, thus reducing NOx emissions [52]. The calorific value of waste transformer oil is lower than that of diesel, and its latent heat of vaporization is higher [48]. This explains why NOx emissions are lower for TD30 samples even though the exhaust temperature is higher.
Exhaust gas temperature (EGT)
Exhaust gas temperatures also increase in compression ignition engines due to the fact that combustion occurs at very high temperatures. It is not desired that the energy released as a result of combustion is discharged from the exhaust as heat energy. The explanation for this situation is that if the temperature of the exhaust gas is high, the thermal efficiency of the compression ignition engine decreases [53].
The changes in the exhaust gas temperatures of the five different fuel samples prepared according to the engine speed are shown in Fig. 12.
When the exhaust gas temperatures in Fig. 12 are examined, it is seen that the exhaust gas temperature increases in direct proportion as a result of the increase in the engine speed for all fuel blends. Due to the presence of water in the biodiesel, increasing of biodiesel in mixtures reduces EGT because the heat of the latent water quenches the chamber due to evaporation [19]. Exhaust gas temperatures for all different fuel blends are higher than for Diesel fuel except for the TD30 fuel blend at 2500 rpm only. Higher exhaust gas temperatures are an indication of a higher work release [53]. When the changes in EGT and NOx are examined, it can be said that EGT and NOx formation are similar only at high speeds (2000 and 2500 rpm).
Conclusions and recommendations
In this study, five different fuel samples were prepared by mixing olive oil wastes biodiesel and waste transformer oil with Euro diesel fuel through transesterification method. First, the thermochemical characterization of the prepared fuel blends was performed, and then, engine performance and exhaust emission tests were conducted on a single-cylinder compression ignition direct injection engine at four different engine speeds (2500, 2000, 1500, and 1000 rpm). The results obtained from this study are summarized briefly in the following points.
-
While the high viscosity and specific gravity values of the olive oil wastes biodiesel and waste transformer oil blend were a disadvantage, their similar characteristics to diesel fuel provided an advantage. It has been observed that all fuel blends meet important fuel properties such as density, viscosity, pour point, and cloud point according to ASTM or EN standards.
-
Euro diesel fuel, waste transformer oil, and biodiesel blends can be used in compression ignition diesel engines without any modifications.
-
The TG-DSC analysis result of the TD30B10 fuel blend confirms its good combustion performance, which is also supported by positive results obtained from engine tests.
-
At all engine speeds, the average torque value produced by each fuel blend was highest for D100 fuel and lowest for TD30 fuel. Among the fuel blends, TD30B10 had the best average torque value with a decrease of 6.48% compared to D100 fuel.
-
At all engine speeds, the average BP produced by each fuel was highest for D100 and lowest for TD30. Among the fuel blends, TD30B10 had the highest average BP value. The maximum BP value for TD30B10 fuel was reached at 2000 rpm.
-
According to the engine experiment, the average specific fuel consumption of all fuel blends produced at all engine speeds was compared with the average specific fuel consumption of D100 fuel at all speeds, and it was found that TD30 fuel blend had a 25.4% increase, TD30B10 fuel blend had an 18% increase, TD30B20 fuel blend had a 20.1% increase, and TD30B30 fuel blend had a 24.8% increase. When these values are examined, the best performing fuel blend is TD30B10.
-
As the engine speed increases, it is generally observed that the CO emission value decreases for all prepared fuel blends. When the fuel blends are compared with the CO emission values of D100 fuel, it is seen that the CO emission value of TD30 fuel blend is high at 1000 and 1500 rpm engine speeds, while it is low at other speeds. The reason for the high CO emission values in all fuel blends at 1000 rpm engine speed is the insufficient air and high temperature combustion, which has increased the CO emission amount at low engine speeds.
-
When the average values of NOx emissions for all fuel blends were compared with the average values of NOx emissions for D100 fuel at all engine speeds, it was determined that there was a 23.98% decrease in TD30 fuel blend, a 14.92% decrease in TD30B10 fuel blend, an 18.78% decrease in TD30B20 fuel blend, and a 14.03% decrease in TD30B30 fuel blend.
The use of waste transformer oil with diesel fuel by mixing it with vegetable waste olive seed biodiesel is important in terms of making the harmful wastes less harmful to the environment by using them in internal combustion engines.
As a suggestion for future studies, the use of end-of-life waste transformer oil together with biodiesels obtained from different vegetable waste oils can be considered. Additionally, different mixtures can be prepared by adding different nanoparticles to binary or ternary mixtures, and the engine performance and exhaust emission parameters of these mixtures can be investigated.
Abbreviations
- ASTM:
-
American Society for Testing and Materials
- VEBD:
-
Vegetable biodiesel
- FAME:
-
Fatty acid methyl ester
- BD:
-
Biodiesel
- WFO:
-
Waste frying oil
- WTO:
-
Waste transformer oil
- RWTO:
-
Refined waste transformer oil
- bTDC:
-
Before top dead center
- BTE:
-
Break thermal efficiency
- BP:
-
Break power
- AFBD:
-
Animal biodiesel
- BSFC:
-
Brake specific fuel consumption
- CCWTO:
-
Catalytic cracked waste transformer oil
- CI:
-
Compression ignition engine
- CA:
-
Crank angle
- DSC:
-
Differential scanning calorimetry analysis
- EGT:
-
Exhaust gas temperature
- EN:
-
European Committee for Standardization
- FTIR:
-
Fourier transform infrared spectroscopy analysis
- GC:
-
Gas chromatography
- T:
-
Torque
- TG:
-
Thermogravimetric analysis
- B100:
-
100% Biodiesel
- T100:
-
100% Waste transformer oil
- D100:
-
100% Euro diesel
- TD30:
-
30% Waste transformer oil, 70% Euro diesel fuel
- TD30B10 :
-
27% Waste transformer oil, 63% Euro diesel fuel, 10% biodiesel
- TD30B20 :
-
24% Waste transformer oil, 56% Euro diesel fuel, 20% biodiesel
- TD30B30 :
-
21% Waste transformer oil, 49% Euro diesel fuel, 30% biodiesel
- °C:
-
Degrees Celsius
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- NOx :
-
Nitrous oxide
- NO:
-
Nitrogen monoxide
- SO2 :
-
Sulfur dioxide
- HC:
-
Hydrocarbon
- UHC:
-
Unburned hydrocarbon
- CH3OH:
-
Methanol
- NaOH:
-
Sodium hydroxide
- rpm:
-
Rounds per minute
- Nm:
-
Newton meter
- g k− 1W− 1 h− 1 :
-
Grams/kilowatt hour
- mL :
-
Milliliter
- kJ k− 1 W− 1 h− 1 :
-
Kilojoule/kilowatt hour
References
Horuz A, Korkmaz A, Akınoğlu G. Biofuel crops and technology. J Soil Sci Plant Nutr. 2015;3(2):69–81.
Saha D, Sinha A, Roy B. A critical review of emission and performance characteristics of CI engine using bio-additives. Clean Technol Environ Policy. 1918;22:1613–38. https://doi.org/10.1007/s10098-020-01918-8.
Acar M, Şahin M. Projection of renewable energy resources (biodiesel, bioethanol and biomass) in Turkey. J Agric Mach Sci. 2012;8(3):337–44.
Simsek S, Uslu S. Determination of a diesel engine operating parameters powered with canola, safflower and waste vegetable oil based biodiesel combination using response surface methodology (RSM). Fuel. 2020. https://doi.org/10.1016/J.FUEL.2020.117496.
Simsek S, Uslu S. Comparative evaluation of the influence of waste vegetable oil and waste animal oil-based biodiesel on diesel engine performance and emissions. Fuel. 2020;280(July): 118613. https://doi.org/10.1016/j.fuel.2020.118613.
Simsek S. Effects of biodiesel obtained from Canola, sefflower oils and waste oils on the engine performance and exhaust emissions. Fuel. 2020. https://doi.org/10.1016/j.fuel.2020.117026.
Utlu Z, Koçak MS. The effect of biodiesel fuel obtained from waste frying oil on direct injection diesel engine performance and exhaust emissions. Renew Energy. 2008;33(8):1936–41. https://doi.org/10.1016/j.renene.2007.10.006.
El-Kassaby M, Nemit-Allah MA. Studying the effect of compression ratio on an engine fueled with waste oil produced biodiesel/diesel fuel. Alexandria Eng J. 2013;52(1):1–11. https://doi.org/10.1016/j.aej.2012.11.007.
Cihan Ö. Experimental and numerical investigation of the effect of fig seed oil methyl ester biodiesel blends on combustion characteristics and performance in a diesel engine. Energy Rep. 2021;7:5846–56. https://doi.org/10.1016/j.egyr.2021.08.180.
Sanli H, Canakci M, Alptekin E, Turkcan A, Ozsezen AN. Effects of waste frying oil based methyl and ethyl ester biodiesel fuels on the performance, combustion and emission characteristics of a di diesel engine. Fuel. 2015;159:179–87. https://doi.org/10.1016/j.fuel.2015.06.081.
Atabani AE, Kulthoom ALS. Spectral, thermoanalytical characterizations, properties, engine and emission performance of complementary biodiesel-diesel-pentanol/octanol blends. Fuel. 2020;282(May):118849. https://doi.org/10.1016/j.fuel.2020.118849.
Karimi P, Najafi B, Ardabili SF, Mesri-Gundoshmian T, Ariyanfar L, Haghighatshoar F. Ethyl ester production from Iranian bitter almond (BAO) oil to improve the performance and emissions of OM457 diesel engine. Renew Energy Focus. 2020;33(June):16–22. https://doi.org/10.1016/j.ref.2020.02.001.
Öztürk E, Can Ö, Usta N, Yücesu HS. Effects of retarded fuel injection timing on combustion and emissions of a diesel engine fueled with canola biodiesel. Eng Sci Technol an Int J. 2020;23(6):1466–75. https://doi.org/10.1016/j.jestch.2020.06.008.
Lakshmana Kumar S, Radjarejesri S, Raj JR. Characterization of waste plastic oil as biodiesel in IC engines. Mater Today Proc. 2020;33:833–8. https://doi.org/10.1016/J.MATPR.2020.06.272.
Mikulski M, Ambrosewicz-Walacik M, Hunicz J, Nitkiewicz S. Combustion engine applications of waste tyre pyrolytic oil. Prog Energy Combust Sci. 2021;85: 100915. https://doi.org/10.1016/j.pecs.2021.100915.
Saha D, Roy B, Pattanayak S, Mishra L, Paban KP. Performance, emission, combustion, exergy, exergoeconomic and sustainability analyses of EGR incorporated CI engine fuelled with areca nut husk nano-additive dosed plastic oil-water-diesel emulsion blend. Therm Sci Eng Prog. 2024;47: 102317. https://doi.org/10.1016/j.tsep.2023.102317.
Saha D, Roy B. Sustainable Energy Technologies and Assessments 54 (2022) 102877 Effects of plastic-grocery-bag derived oil-water-diesel emulsions on combustion, performance and emission characteristics, and exergoeconomic aspects of compression ignition engine. Published online 2022. https://doi.org/10.1016/j.seta.2022.102877
Saha D, Roy B. Plastic-grocery-bag-derived oil and its emulsion for compression ignition engine application: emulsion characteristics and combustion-performance-emission analysis. J Therm Anal Calorim. 2023;148:13929–40. https://doi.org/10.1007/s10973-023-12700-5.
Saha D, Roy B. Influence of areca nut husk nano-additive on combustion, performance, and emission characteristics of compression ignition engine fuelled with plastic-grocery-bag derived oil-water-diesel emulsion. Energy. 2023. https://doi.org/10.1016/j.energy.2023.126682.
Santhoshkumar A, Ramanathan A. Recycling of waste engine oil through pyrolysis process for the production of diesel like fuel and its uses in diesel engine. Energy. 2020. https://doi.org/10.1016/J.ENERGY.2020.117240.
Sathish T, Surakasi R, KishoreT L, et al. Waste to fuel: pyrolysis of waste transformer oil and its evaluation as alternative fuel along with different nanoparticles in CI engine with exhaust gas recirculation. Energy. 2023. https://doi.org/10.1016/J.ENERGY.2022.126595.
Nabi MN, Akhter MS, Rahman MA. Waste transformer oil as an alternative fuel for diesel engine. Procedia Eng. 2013;56:401–6. https://doi.org/10.1016/j.proeng.2013.03.139.
Yadav SPR, Saravanan CG, Kannan M. Influence of injection timing on di diesel engine characteristics fueled with waste transformer oil. Alexandria Eng J. 2015;54(4):881–8. https://doi.org/10.1016/j.aej.2015.07.008.
Belkhode PN, Ganvir VN, Shende AC, Shelare SD. Utilization of waste transformer oil as a fuel in diesel engine. Mater Today Proc. 2021;49:262–8. https://doi.org/10.1016/j.matpr.2021.02.008.
Öner İV, Atabani AE, Durnagöl T. Recycling of waste tires to crude pyrolytic oil: Engine performance, combustion characteristics and emissions analysis of diesel-butanol-crude pyrolytic oil blends in CI diesel engines. Sustain Energy Technol Assessm. 2023. https://doi.org/10.1016/j.seta.2023.103023.
Basha SA, Gopal KR, Jebaraj S. A review on biodiesel production, combustion, emissions and performance. Renew Sustain Energy Rev. 2009;13(6–7):1628–34. https://doi.org/10.1016/j.rser.2008.09.031.
Balat M, Balat H. Progress in biodiesel processing. Appl Energy. 2010;87(6):1815–35. https://doi.org/10.1016/j.apenergy.2010.01.012.
Prasanna Raj Yadav S, Saravanan CG, Vallinayagam R, Vedharaj S, Roberts WL. Fuel and engine characterization study of catalytically cracked waste transformer oil. Energy Convers Manag. 2015;96:490–8. https://doi.org/10.1016/j.enconman.2015.02.051.
Ajay J, Viswanath G. A study on waste transformer oil blended with BD IC engine application. Mater Today Proc. 2020;22:865–8. https://doi.org/10.1016/j.matpr.2019.11.034.
Sethuraman N, Karthikeyan D, Vinodraj S, Thamizhvel R, Kumarasamy G. Performance and emission characteristics on CI engine by using waste cooking oil and waste transformer oil as substitute fuel with coconut shell as a catalyst. Mater Today Proc. 2022;72:2469–75. https://doi.org/10.1016/j.matpr.2022.09.457.
Singh R, Kumar R. Insights into the influence of n-butanol with neat biodiesel and biodiesel-diesel blends on diesel engine characteristics: review. Int J Energy Res. 2022;46(5):5441–66. https://doi.org/10.1002/er.7550.
Öztürk E. Performance, emissions, combustion and injection characteristics of a diesel engine fuelled with canola oil-hazelnut soapstock biodiesel mixture. Fuel Process Technol. 2015;129:183–91. https://doi.org/10.1016/j.fuproc.2014.09.016.
Dehghan L, Golmakani MT, Hosseini SMH. Optimization of microwave-assisted accelerated transesterification of inedible olive oil for biodiesel production. Renew Energy. 2019;138:915–22. https://doi.org/10.1016/j.renene.2019.02.017.
Atabani AE, Mekaoussi M, Uguz G, Arpa O, Ayanoglu A, Shobana S. Evaluation, characterization, and engine performance of complementary fuel blends of butanol–biodiesel–diesel from Aleurites moluccanus as potential alternative fuels for CI engines. Energy Environ. 2020;31(5):755–84. https://doi.org/10.1177/0958305X18790953.
Abdelrahman MH, Hussain RO, Shaheed DS, Abukhader M, Khan SA. Gas chromatography-mass spectrometry analysis and in vitro biological studies on fixed oil isolated from the waste pits of two varieties of Olea europaea L. OCL-Oilseeds fats. Crop Lipids. 2019;26(4):1–8. https://doi.org/10.1051/ocl/2019022.
Bedir Ö, Doğan TH. Comparison of catalytic activities of Ca-based catalysts from waste in biodiesel production. Energy Sour Part A Recover Util Environ Eff. 2021;00(00):1–18. https://doi.org/10.1080/15567036.2021.1883159.
Sharma V, Ganesh D. Combustion and emission characteristics of reformulated biodiesel fuel in a single-cylinder compression ignition engine. Int J Environ Sci Technol. 2020;17(3):243–52. https://doi.org/10.1007/s13762-019-02285-8.
Bukkarapu KR, Krishnasamy A. A study on the effects of compositional variations of biodiesel fuel on its physiochemical properties. Biofuels. 2021;12(5):523–35. https://doi.org/10.1080/17597269.2018.1501638.
Plasquy E, García Martos JM, Florido Fernández MDC, Sola-Guirado RR, García Martín JF. Cold storage and temperature management of olive fruit: the impact on fruit physiology and olive oil quality—a review. Processes. 2021. https://doi.org/10.3390/pr9091543.
Mohta V, Basavaraj M. Preparation of alternative fuel from waste transformer oil and studying performance charachteristics on diesel engine for different blends. Int Res J Eng Technol. 2015;4(10):2013–6.
Ayadi M, Saragih FNA, Awad S, et al. Two steps methanolysis and ethanolysis of olive pomace oil using olive-pomace-based heterogeneous acid catalyst. Fuel. 2021;296(March): 120678. https://doi.org/10.1016/j.fuel.2021.120678.
Al-Samaraae R, Atabani A, Uğuz G, et al. Perspective of safflower ( Carthamus tinctorius ) as a potential biodiesel feedstock in Turkey: characterization, engine performance and emissions analyses of butanol–biodiesel–diesel blends. Biofuels. 2017;11:1–17. https://doi.org/10.1080/17597269.2017.1398956.
Dharma S, Silitonga AS, Shamsuddin AH, et al. Properties and corrosion behaviors of mild steel in biodiesel-diesel blends. Energy Sour Part A Recover Util Environ Effects. 2019;00(00):1–13. https://doi.org/10.1080/15567036.2019.1668883.
Shehata MS, Razek SMA. Experimental investigation of diesel engine performance and emission characteristics using jojoba/diesel blend and sunflower oil. Fuel. 2010. https://doi.org/10.1016/j.fuel.2010.09.011.
Tekbaş ÖF, Vaizoğlu S, Oğur R, Güler Ç. Global Warming, Climate Change and Health Impacts. Ankara. Published online 2005:49–50
Ruhul AM, Kalam MA, Masjuki HH, et al. Evaluating combustion, performance and emission characteristics of Millettia pinnata and Croton megalocarpus biodiesel blends in a diesel engine. Energy. 2017;141:2362–76. https://doi.org/10.1016/J.ENERGY.2017.11.096.
Agarwal D, Kumar AA. Performance and emissions characteristics of Jatropha oil (preheated and blends) in a direct injection compression ignition engine. Appl Thermal Eng. 2007. https://doi.org/10.1016/j.applthermaleng.2007.01.009.
Behera P, Murugan S. Combustion, performance and emission parameters of used transformer oil and its diesel blends in a DI diesel engine. Fuel. 2013;104:147–54. https://doi.org/10.1016/j.fuel.2012.09.077.
Haşimoğlu C, İçingür Y, Öğüt H. Experimental analysis of the effect of exhaust gas recirculation (EGR) on engine performance and exhaust emissions in diesel engines. Turk J Eng Environ Sci. 2002;26(2):127–35.
İlkılıç C, Behçet R, Aydın S. NOx formation in diesel engines and control methods. 5th Int Adv Technol Symp. Published online 2009:1–5.
Rao YVH, Voleti RS, Raju AVS, Reddy PN. The effect of cottonseed oil methyl ester on the performance and exhaust emissions of a diesel engine. Int J Ambient Energy. 2011. https://doi.org/10.1080/01430750.2010.9675813.
Yusuf AA, Dankwa Ampah J, Veza I, et al. Investigating the influence of plastic waste oils and acetone blends on diesel engine combustion, pollutants, morphological and size particles: dehalogenation and catalytic pyrolysis of plastic waste. Energy Convers Manag. 2023;291: 117312. https://doi.org/10.1016/j.enconman.2023.117312.
Arpa O, Demir M, Yumrutaş R. Investigation of engine performance and exhaust emissions of fuel-diesel mixtures obtained from waste lubricating oil. Dicle Univ Eng Fac J Eng. 2019;10(1):191–201. https://doi.org/10.24012/dumf.523585.
Acknowledgements
This article represents the output of the Masters degree thesis of Mr. Muhammet Büyükoglu (Atatürk University,Thesis number: 808168) (Higher Education Board of Türkiye, YÖK).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). Not applicable.
Author information
Authors and Affiliations
Contributions
MB contributed to methodology, material preparation, data collection, analysis, and writing. THD contributed to conceptualization, data analysis, writing, reviewing, and editing, and methodology. OA contributed to conceptualization, formal analysis, data collection and analysis, and writing. HN contributed to conceptualization, data analysis, formal analysis, reviewing, and writing. İVÖ contributed to methodology, investigation, conceptualization, data curation, formal analysis, supervision, validation, visualization, and writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Availability of data and materials
Data will be made available on request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Büyükoğlu, M., Doğan, T.H., Arpa, O. et al. Experimental investigation of engine performance and emissions, and characterization, of waste transformer oils and diesel blends with biodiesel produced from olive oil wastes in a CI engine. J Therm Anal Calorim 149, 5381–5398 (2024). https://doi.org/10.1007/s10973-024-13207-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-024-13207-3