Abstract
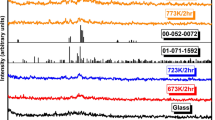
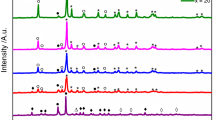
This paper deals with the crystallization kinetics, optical and structural features of V2−xMgxO5−δ (x = 0.15–0.30) glasses under non-isothermal conditions using Lasocka, Kissinger and Augis-Bennett approaches. Raman spectra confirm the layer structure of vanadium in the present glasses. The optical band gap decreases from 2.64 to 2.20 eV with an increase in MgO doping, while refractive index (n) increases from 2.50 to 2.65 with an increase in MgO doping. The activation energy of glass transition and crystallization increase from 150 to 229 kJ mol−1 and 256–442 kJ mol−1, respectively, with the doping of MgO in V2O5. The kinetic parameters calculated from different approaches are found to be in a good agreement with each other. The fragility index increases from 29 to 51 with increasing MgO content in place of V2O5. The values of \({f}_{\text{g}}\) and \({E}_{\text{c}}\) are found to enhance with changes in heating rates due to the strong influence of heating rates on glass transition temperature.













Similar content being viewed by others
References
Issever U, Kilic G, Ilik E. The impact of CuO on physical, structural, optical and thermal properties of dark VPB semiconducting glasses. Opt Mater. 2021;116:111084. https://doi.org/10.1016/j.optmat.2021.111084.
Khattak G, Mekki A, Wenger L. X-ray photoelectron spectroscopy (XPS) and magnetic susceptibility studies of vanadium phosphate glasses. J Non-Cryst Solids. 2019;355(43–44):2148–55. https://doi.org/10.1016/j.jnoncrysol.2009.06.042.
Montani RA, Frechero MA. Mixed ion-polaron transport in lithium vanadium–molybdenum tellurite glasses. Solid State Ionics. 2006;177(33–34):2911–5. https://doi.org/10.1016/j.ssi.2006.08.015.
Salehizadeh S, Melo B, Freire F, Valente M, Graca M. Structural and electrical properties of TeO2–V2O5–K2O glassy systems. J Non-Cryst Solids. 2016;443:65–74. https://doi.org/10.1016/j.jnoncrysol.2016.03.012.
Hoppe U, Yousef E, Russel C, Neuefeind J, Hannon A. Structure of vanadium tellurite glasses studied by neutron and x-ray diffraction. Solid State Commun. 2002;123(6–7):273–8. https://doi.org/10.1016/S0038-1098(02)00303-4.
Hirashima H, Mitsuhashi M, Yoshida T. Electrical conduction of Fe2O3-V2O5-P2O5 glasses. J Ceram Soc Jpn. 1982;90(4):411–9.
Hirashima H, Kurokawa H, Mizobuchi K, Yoshida T. Electrical conductivity of vanadium phosphate glasses containing ZnO or GeO2. Glass Sci Tech. 1988;61(6):151–6.
Ghosh A, Chaudhuri B. DC conductivity of V2O5-Bi2O3 glasses. J Non-Cryst Solids. 1986;83(1–2):151–61. https://doi.org/10.1016/0022-3093(86)90065-7.
Pawaria S, Bala M, Duhan H, Deopa N, Dahiya S, Ohlan A, Punia R, Maan AS. Study of crystallization and glass transition kinetics of bismuth-modified zinc vanadate glasses by non-isothermal method. J Therm Anal Calorim. 2022;147(23):13099–110. https://doi.org/10.1007/s10973-022-11531-0.
Pawaria S, Ahlawat J, Sharma P, Dahiya S, Ohlan A, Punia R, Maan AS. Glass transition and crystallization kinetics of lithium modified zinc borate semiconducting glasses by non-isothermal method. Ceram Int. 2023;49(14):23276–86. https://doi.org/10.1016/j.ceramint.2023.04.158.
Shaaban E, Hawary ME, Salami AEA, Assiri MA. Crystallization kinetics of semiconducting vanadium borate glass using DSC. Phys Scr. 2010;82(4):045603. https://doi.org/10.1088/0031-8949/82/04/045603.
Danewalia SS, Singh K, Arya SK. Influence of vanadium oxide on non-isothermal crystallization kinetics of zinc lithium borate glasses. J Non-Cryst Solids. 2021;553:120471. https://doi.org/10.1016/j.jnoncrysol.2020.120471.
Subhadra M, Sulochana S, Kistaiah P. Effect of V2O5 content on physical and optical properties of lithium bismuth borate glasses. In: Proceedings Material Today. 2018; 5 (13): 26417–26423. Doi: https://doi.org/10.1016/j.matpr.2018.08.095
Margha FH, Marzouk M. Influence of vanadium addition on the optical and photoluminescence properties of borate glasses and their glass–ceramic derivatives. Appl Phys A. 2019;125:1–9. https://doi.org/10.1007/s00339-019-2922-0.
Lahl N, Singh K, Singheiser L, Hilpert K, Bahadur D. Crystallisation kinetics in AO-Al2O3-SiO2-B2O3 glasses (A= Ba, Ca, Mg). J Mater Sci. 2000;35:3089–96. https://doi.org/10.1023/A:1004851418274.
Kaur M, Kaur G, Pandey OP, Singh K, Kumar V. Influence of CaO/MgO ratio on the crystallization kinetics and interfacial compatibility with crofer 22APU and YSZ of strontium based alumino-borosilicate glasses for SOFC applications. Int J Hydrog Energy. 2017;42(25):16244–57. https://doi.org/10.1016/j.ijhydene.2017.05.026.
Lee SH, Cheong HM, Seong MJ, Liu P, Tracy CE, Mascarenhas A, Pitts JR, Deb SK. Raman spectroscopic studies of amorphous vanadium oxide thin films. Solid State Ion. 2003;165(1–4):111–6. https://doi.org/10.1016/j.ssi.2003.08.022.
Sethi D, Jada N, Tiwari A, Ramasamy S, Dash T, Pandey S. Photocatalytic destruction of escherichia coli in water by V2O5/TiO2. J Photochem Photobiol B: Biol. 2015;144:68–74. https://doi.org/10.1016/j.jphotobiol.2015.02.003.
Zhan S, Chen G, Liu D, Li A, Wang C, Wei Y. Effects of Cr doping on the structural and electrochemical properties of V2O5. J Alloys Compd. 2009;479(1–2):652–6. https://doi.org/10.1016/j.jallcom.2009.01.023.
Bhaskaram DS, Cheruku R, Govindaraj G. Reduced graphene oxide wrapped V2O5 nanoparticles: green synthesis and electrical properties. J Mater Sci Mater Electron. 2016;27:10855–63. https://doi.org/10.1007/s10854-016-5194-x.
Khan S, Singh K. Influence of Al3+ doping for V5+ on the structural, optical, thermal and electrical properties of V2-xAlxO5-δ (x= 0–0.20) ceramics. Ceram Int. 2021;47(8):10724–32. https://doi.org/10.1016/j.ceramint.2020.12.188.
Souri D, Tahan ZE. A new method for the determination of optical band gap and the nature of optical transitions in semiconductors. Appl Phys B. 2015;119(2):273–9. https://doi.org/10.1007/s00340-015-6053-9.
Souri D, Sarfehjou M, Khezripour AR. The effect of ambient temperature on the optical properties and crystalline quality of ZnSe and ZnSe: Cu NCs grown by rapid microwave irradiation. J Mater Sci: Mater Electron. 2018;29:3411–22. https://doi.org/10.1007/s10854-017-8276-5.
Souri D, Mohammadi M, Zaliani H. Effect of antimony on the optical and physical properties of Sb-V 2 O 5-TeO 2 glasses. Electron Mater Lett. 2014;10:1103–8. https://doi.org/10.1007/s13391-014-4047-0.
Alazoumi SH, Aziz SA, Mallawany RE, Aliyu US, Kamari HM, Zaid MHMM, Matori KA, Ushah A. Optical properties of zinc lead tellurite glasses. Results Phys. 2018;9:1371–6. https://doi.org/10.1016/j.rinp.2018.04.041.
Kumar S, Singh K, Kumar D. Asymmetric SiO2 structural units modification by Li2O and their effect on optical and mechanical properties of soda lime silicate glasses. Ceram Int. 2023;49(16):26302–12. https://doi.org/10.1016/j.ceramint.2023.05.143.
Elkhoshkhany N, Reda A, Embaby AM. Preparation and study of optical, thermal, and antibacterial properties of vanadate–tellurite glass. Ceram Int. 2017;43(17):15635–44. https://doi.org/10.1016/j.ceramint.2017.08.120.
Khan S, Kaur G, Singh K. Effect of ZrO2 on dielectric, optical and structural properties of yttrium calcium borosilicate glasses. Ceram Int. 2017;43:722–7. https://doi.org/10.1016/j.ceramint.2016.09.219.
Kumar S, Kumar D, Singh K. Modification of silicate structural units by K2O for enhancing automobile windshield glass properties. J Phys Chem Solids. 2023;181:111523. https://doi.org/10.1016/j.jpcs.2023.111523.
Rao SLS, Ramadevudu G, Shareefuddin M, Hameed A, Chary MN, Rao ML. Optical properties of alkaline earth borate glasses. Int J Eng Sci. 2012;4(4):25–35. https://doi.org/10.4314/ijest.v4i4.3.
Berwal N, Kundu R, Nanda K, Punia R, Kishore N. Physical, structural and optical characterizations of borate modified bismuth–silicate–tellurite glasses. J Mol Struct. 2015;1097:37–44. https://doi.org/10.1016/j.molstruc.2015.05.011.
Saeed A, Bashar YE, Kameesy SE. Optical spectroscopic analysis of high-density lead borosilicate glasses. SILICON. 2018;10:185–9. https://doi.org/10.1007/s12633-015-9391-7.
Joshi S, Pratap A, Saxena N, Saksena M, Kumar A. Heating rate and composition dependence of the glass transition temperature of a ternary chalcogenide glass. J Mater Sci lett. 1994;13:77–9. https://doi.org/10.1007/BF00416803.
Moynihan CT, Easteal AJ, Wilder J, Tucker J. Dependence of the glass transition temperature on heating and cooling rate. J Phys Chem. 1974;78(26):2673–7. https://doi.org/10.1021/j100619a008.
Khan S, Singh K. Effect of MgO on structural, thermal and conducting properties of V2-xMgxO5-δ (x=0.05–0.30) systems. Ceram Int. 2019;45:695–701. https://doi.org/10.1016/j.ceramint.2018.09.231.
Khan S, Singh K. Effect of TiO2 doping on structural and electrical properties of melt-quench of V2-xTixO5-δ, (0.15 ≤ x ≤0.30) systems. J Mater Sci: Mater Electron. 2021;32:12594–607. https://doi.org/10.1007/s10854-021-05896-5.
Khan S, Singh K. Structural, optical, thermal and conducting properties of V2-xLixO5-δ (0.15≤x≤0.30). Sci Rep. 2021;10:1089. https://doi.org/10.1038/s41598-020-57836-8.
Mandal S, Ghosh A. Structure and physical properties of glassy lead vanadates. Phys Rev B. 1993;48(13):9388. https://doi.org/10.1103/PhysRevB.48.9388.
Sen S, Ghosh A. Structural properties of strontium vanadate glasses. J Mater Res. 2000;15(4):995–9. https://doi.org/10.1557/JMR.2000.0142.
Hajry AA, Shahrani AA, Desoky ME. Structural and other physical properties of barium vanadate glasses. Mater Chem Phys. 2006;95(2–3):300–6. https://doi.org/10.1016/j.matchemphys.2005.06.041.
Alencar MV, Bezerra GV, Silva LD, Schneider JF, Pascual MJ, Cabral AA. Structure, glass stability and crystallization activation energy of SrO-CaO-B2O3-SiO2 glasses doped with TiO2. J Non-Cryst Solids. 2021;554:120605. https://doi.org/10.1016/j.jnoncrysol.2020.120605.
Kissinger HE. In differential thermal analysis. J Res Natl Bur. 1956;57(4):217.
Augis J, Bennett J. Calculation of the Avrami parameters for heterogeneous solid-state reactions using a modification of the Kissinger method. J Therm Anal. 1978;13:283–92. https://doi.org/10.1007/BF01912301.
Hosseini SF, Souri D, Nouri EA. Functional thermal stable samples: non-isothermal calorimetric analysis of MoO3–V2O5–TeO2 oxide glasses. J Inorg Organomet Polym Mater. 2021;31:2877–90. https://doi.org/10.1007/s10904-021-01911-8.
Kaur G, Pandey OP, Singh K. Microstructural analysis of interfaces between lanthanum contained glass and two different electrolytes for SOFC applications. Fuel Cells. 2012;12(5):739–48. https://doi.org/10.1002/fuce.201200080.
Souri D, Zaliani H, Mirdawoodi E, Zendehzaban M. Thermal stability of Sb–V2O5–TeO2 semiconducting oxide glasses using thermal analysis. Measurement. 2016;82:19–25. https://doi.org/10.1016/j.measurement.2015.12.026.
Souri D, Shahmoradi Y. Calorimetric analysis of non-crystalline TeO2–V2O5–Sb2O3: determination of crystallization activation energy, Avrami index and stability parameter. J Therm Anal Calorim. 2017;129:601–7. https://doi.org/10.1007/s10973-017-6151-5.
Hosseini SF, Souri D. Nouri EA functional thermal stable samples: non-isothermal calorimetric analysis of MoO3–V2O5–TeO2 oxide glasses. J Inorg Organomet Polym Mater. 2021;31:2877–90. https://doi.org/10.1007/s10904-021-01911-8.
Souri D. Physical and thermal characterization and glass stability criteria of amorphous silver-vanadate-tellurate system at different heating rates: Inducing critical Ag2O/V2O5 ratio. J Non-Cryst Solids. 2017;475:136–43. https://doi.org/10.1016/j.jnoncrysol.2017.09.008.
Nichenko TM, Rizak V, Nichenko TM, Fedelesh V. Parameters of the fluctuation free volume theory for glasses in the Ge-As-Se system. Glass Phys Chem. 2004;30(5):406–14. https://doi.org/10.1023/B:GPAC.0000045920.01447.ba.
Tyurnina N, Tyurnina Z, Sviridov S. Density and microhardness of glasses in the SrO-B2O3-SiO2 system. Glass Phys Chem. 2009;35:153–7. https://doi.org/10.1134/S1087659609020059.
Bainova A, Sanditov D. Dependence of the fluctuation free volume of amorphous substances on the cooling rate. Glass Phys Chem. 2002;28:189–90. https://doi.org/10.1023/A:1016007503820.
Ojha PK, Rath SK, Sharma SK, Sudarshan K, Pujari PK, Chongdar TK, Gokhale NM. Free volume of mixed cation borosilicate glass sealants elucidated by positron annihilation lifetime spectroscopy and its correlation with glass properties. J Power Sources. 2015;273:937–44. https://doi.org/10.1016/j.jpowsour.2014.10.005.
Acknowledgements
One of the authors (Savidh Khan) thanks Haya Khan, Department of Chemistry, School of Natural Sciences, Shiv Nadar Institutions of Eminence (Deemed to be University), Dadri, Gautam Budh Nagar-201314, UP, India, for her help during UV-visible characterization.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, S., Kumar, S., Abida, K. et al. Non-isothermal crystallization kinetics, optical and structural properties of MgO-doped vanadate glasses. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13173-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13173-w