Abstract
Precipitation processes in age hardenable aluminium alloys are often investigated by differential scanning calorimetry (DSC). The endothermic and exothermic peaks of the DSC signal correspond to the dissolution and formation of phases, respectively. However, parasitic effects can lead to an unintended curvature of the DSC signal. Although a baseline correction can be used, some imperfections typically remain. Additionally, sample preparation and experimental conditions can influence the precipitation sequence itself and, therefore, the DSC curve. In this study, we investigate the influence of sample preparation by milling and punching on DSC curves for three different aluminium alloys: EN AW-2024, EN AW-6082, and EN AW-7075. Additionally, the influence of quenching and natural ageing was investigated for EN AW-6082. We found that deformation introduced by punching the DSC samples with a piercing die after heat treatment leads to a change in the precipitation kinetics in 2xxx, 6xxx, and 7xxx series alloys. The influence was strongest for punching the samples after solution heat treatment and less significant for punching after artificial ageing. The influence of sample preparation can be avoided by punching the samples before solution heat treatment. If this is not practicable, milling of the samples is a good alternative. The choice of quenching medium and short storage at room temperature before measurement (5 min) had only small effects on the DSC curves. Moreover, the start temperature of the measurement was found to be crucial. For observing phases forming below 100 °C and for low-bias baseline correction, the measurement should start at low-temperatures (i.e. \(\sim\) − 50 °C).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Age-hardenable aluminium alloys are an important material class and extensively used in the transportation sector and other applications [1, 2]. Alloys of the 2xxx and 7xxx series are predominantly used in aircraft construction, either as components milled from plates (e.g. stringers), or as sheets for the outer skin [3, 4]. Alloys of the 6xxx series are often used in the automotive industry as extruded profiles, forged parts, or formed sheet components due to their favourable processability by extrusion, forging, and rolling [5,6,7] and good room temperature formability by processes such as deep drawing or bending [8].
Heating and cooling of aluminium alloys according to a specific time-temperature curve is essential to obtain desired material properties, which go along with the precipitation kinetics, i.e. the evolution of phases. The material properties are mainly driven by age hardening.
Precipitation in age-hardenable aluminium alloys
Age hardening is based on the principle of decreasing the solubility of alloying elements in the aluminium matrix at lower temperatures [9]. It is essential to first achieve a supersaturated solid solution (SSSS), which can be realised by solution heat treatment (SHT) followed by quenching. The quenching is usually accomplished using water for fast cooling and air for moderate cooling. Subsequent storage at room temperature, known as natural ageing (NA), leads to the precipitation of nanoscale phases, which significantly increase the strength of the material [10,11,12,13]. This process can be strongly accelerated by a heat treatment at increased temperatures (roughly 100–200 °C, alloy-dependent), known as artificial ageing (AA) [14,15,16]. Solution heat treatment, quenching, natural ageing, and artificial ageing are characterised by specific process parameters, which have a strong influence on the precipitation of particles and thus on the material properties.
During the early stages of natural or artificial ageing, solute clusters of the main alloying elements start forming from the SSSS [13, 17,18,19,20]. When periodic ordering occurs within the clusters at a later stage and coherency with the aluminium matrix still exists, they are known as Guinier–Preston zones (GP-zones) in 6xxx [21] and 7xxx [22], or also as Guinier–Preston–Bagaryatsky zones (GPB-zones) in 2xxx series alloys [23]. During artificial ageing or in some cases of very long natural ageing [12], partially coherent, thermodynamically metastable precipitates form, such as \(\beta ''\) in 6xxx series [14, 24, 25], \(\theta '\) in 2xxx series [26], or \(\eta '\) in 7xxx series [27, 28]. Together with GP-zones, these precipitates are responsible for the high strength at the peak-aged state. Finally, for long durations of artificial ageing or at higher temperatures, the thermodynamic equilibrium phases, such as \(\beta\) or Q in 6xxx series, \(\theta\) in 2xxx series and \(\eta\) in 7xxx series, form. These equilibrium phases are generally incoherent with the matrix and can be mainly found in over-aged microstructures. However, exceptions apply, with the equilibrium S-phase being an important hardening phase in EN AW-2024-T6 [16, 29]. In summary, a simplified and general precipitation sequence for many wrought aluminium alloys can be given as:
SSSS \(\rightarrow\) coherent clusters/GP-zones \(\rightarrow\) metastable, partially coherent phases \(\rightarrow\) incoherent equilibrium phases.
The respective precipitation sequences of the 2xxx, 6xxx, and 7xxx series alloys used in this work, which have emerged over years of research, are well described in the literature: EN AW-2024 [15, 29, 30], EN AW-6082 [18, 31,32,33,34], and EN AW-7075 [35,36,37,38].
Differential scanning calorimetry (DSC) is suitable for studying the precipitation kinetics of aluminium alloys. Even the formation and dissolution of nanoscale phases such as GP-zones and atomic clustering can be analysed by DSC [15, 18, 39]. Compared to other analytical techniques capable of studying these phenomena, such as atom probe tomography (APT), transmission electron microscopy (TEM), and small-angle X-ray spectroscopy (SAXS), DSC is very inexpensive and, consequently, more widely available. Further advantages of DSC are simple sample preparation, ease of handling, and precise temperature control.
Differential scanning calorimetry of aluminium alloys
DSC determines the heat flow \({\dot{Q}}\) of a sample at a constant heating rate \({\dot{T}}\) within a predefined temperature range. Using the sample mass m, the specific heat capacity c of the sample can be calculated by
Aluminium alloys are multi-phase systems, where each phase i is characterised by its phase fraction \(x_i \in [0,1]\) and its specific heat capacity \(c_i\) depending on the temperature T. Considering N phases, the specific heat capacity c follows as
In case of reactions, \(c_i\) is usually shown as [40, 41]
The first term in (3) is the sensible heat capacity \(c^\text{s}_\text{i}(T)\) driven by the external temperature change and the second one is the latent heat capacity \(c^\text{l}_\text{i}\) related to the released or dissolved energy of the phase transformation within the material. Note that \(c^\text{s}_\text{i}(T)\) is monotonously increasing, i.e. \(\textrm{d}c_\text{i}^\text{s}(T)/\textrm{d} T \ge 0\), and \(c^\text{l}_\text{i}\) is constant. Equation (2) and (3) yield
Here, \({\textbf{x}}= \left[ x_1,...,x_{{\rm N}}\right] ^\top\) is the vector of the phase fractions of all phases in the material. DSC yields information about the formation and dissolution of second-phase precipitates (precipitation sequences) depending on the temperature, cf. (4).
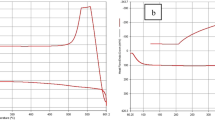
Principle of heat-flux DSC measurement, adapted after [42]: (a) Measurement of \(\Delta T\) between the crucibles of the sample and the reference (empty), (b) recorded temperatures of the sample and the reference. The transition enthalpy of a reaction \(\Delta H\) can be correlated to the temperature difference \(\Delta T\)
Common modes of DSC measurement are heat-flux DSC and power-compensated DSC [43]. The principle of heat-flux DSC measurement, as used in this work, is shown in Fig. 1a. Two crucibles, one empty and one containing the sample to be examined, are heated simultaneously and the temperature difference is recorded by temperature sensors. While the temperature in the empty crucible increases steadily, endothermic or exothermic reactions cause a deviation in temperature in the crucible containing the sample. The measured temperature difference can be converted into a heat flow signal \({\dot{Q}}\), as shown in Fig. 1b.
For power-compensated DSC, another arrangement of the crucibles is used. Both crucibles are filled with sample material (reference and sample to be investigated) and these are heated at the same rate in two separate chambers. The difference in the dissipated electrical power is used to calculate the heat flow \({\dot{Q}}\).
The precipitation kinetics cannot be determined directly from the DSC signal (1). Therefore, the DSC signal must be preprocessed to extract the latent heat capacity \(c^\text{l}({\textbf{x}})\). In this context, each deviation from \(c^\text{l}({\textbf{x}})\) is denoted as the baseline of the measurement, which comprises the sensible heat capacity \(c^\text{s}({\textbf{x}},T)\) and parasitic effects, see "Parasitic effects in DSC" section. If \(c^\text{l}({\textbf{x}})\) is known, the evolution of the phase fractions \(x_\text{i}\) depending on the temperature T can be analysed by interpretation of \(c^\text{l}({\textbf{x}})\) , since \(c^\text{l}_\text{i}\) is constant. However, the interpretation, especially quantitatively, is complicated due to parasitic effects and the complex precipitation kinetics of aluminium alloys. Although baseline correction can be used to reduce the influence of parasitic effects, the correction also has the potential to introduce additional errors or biases, see “Baseline correction of DSC curves” section. The precipitation kinetics of wrought aluminium alloys lead to overlapping peaks in \(c^\text{l}({\textbf{x}})\). Therefore, the interpretation is non-trivial and requires either additional microstructural investigations, or one has to rely on the literature (e.g. [17, 36, 39, 44, 45]).
Calibration process in DSC
To obtain high-quality data in DSC, it is essential to perform a calibration routine. The specific calibration routines may differ for various instrument types [46], but generally consist of a temperature calibration as well as a caloric calibration. The temperature calibration is used to correlate the measured temperature \(T_\text{m}\) to the actual temperature \(T_0\). This is necessary because \(T_0\) might differ from \(T_\text{m}\) either due to thermal gradients or inaccuracies of the sensor itself [47, 48]. Therefore, the measured temperature has to be corrected according to
with a correction function \(\Delta T_\text{corr}\) depending on the temperature \(T_\text{m}\) and heating rate \({\dot{T}}\). Caloric calibration, on the other hand, is used to establish a relationship between the measured signal \(V_\text{m}\) and the heat flow \({\dot{Q}}\) absorbed or released by the sample [47]
where \(K(T_\text{m})\) is the caloric sensitivity function of the sensor that has to be calibrated [48]. For a heat-flux DSC, \(V_\text{m}\) corresponds to the temperature difference between the two crucibles, whereas for a power-compensated DSC, \(V_\text{m}\) corresponds to the electrical power.
For the calibration of the heating rate of 10 K min−1 we used the pure elements indium, bismuth, tin, zinc, and aluminium with different known melting points and enthalpies and evaluated the corresponding curves. None of the elements used for calibration show allotropic transformation in the relevant temperature ranges (i.e. RT to melting point). An example of the curve evaluation is shown in Fig. 2. The melting peak of pure aluminium is clearly delimited and no further precipitation occurs. Therefore, the peak evaluation (onset temperature and peak area) is straightforward.
To further minimise errors of the device and crucible, an additional correction measurement can be carried out with two empty crucibles, known as zeroline measurement [43]. The influence of the crucible material can thus be minimised. The measurements are then performed at the same settings and the correction curve can be automatically subtracted to avoid effects that do not come from the sample [49].
Constant heating and cooling stresses the sensor, so that the actual temperature (5) and the sensitivity \(K(T_\text{m})\) in (6) must be recalibrated at reasonable intervals to obtain high-quality data in DSC. Nevertheless, each DSC measurement is compromised by parasitic effects resulting in a bent baseline [50].
Parasitic effects in DSC
Parasitic effects refer to inaccuracies in the actual temperature (5) and heat flow (6) due to a nonideal measurement setup like the placement of the thermocouples measuring the temperature difference \(\Delta T\) between the two crucibles [51] and heat exchange by radiation.
The thermocouples cannot be placed directly in the investigated samples but are rather located on the outer surface of the crucibles. Therefore, a signal delay caused by the heat transfer through the wall of the crucibles cannot be avoided. This signal delay depends on the material of the crucibles as well as the thermal properties of the sample. Besides that, heat exchange by radiation plays an important role when measurements at higher temperatures are carried out. While a pure aluminium sample keeps its shiny surface during the measurement, alloys can oxidise and darken the surface of the sample [50]. This leads to higher radiation losses in the alloyed sample compared to the reference sample, which has a very high impact on the baseline stability, as shown in [52]. Furthermore, Birol [53] showed that the sample preparation prior to the measurement, e.g. milling or punching, also has a significant influence on the outcome of the measurement. Generally, there are many more parameters that can affect the heat flow \({\dot{Q}}\) determined by the DSC device. For example, the sample mass, the surface-area-to-volume ratio of the sample, the volume of the crucible taken up by the sample, the position of the sample within the crucible, and so on [54].
Baseline correction of DSC curves
In general, the heat capacity c determined by the DSC, cf. (1), is superimposed by a measurement error \(\varepsilon\) due to parasitic effects, which have to be handled in a proper manner. By using (4), this yields
where the measurement error depends on the actual temperature \(T_0\).
As mentioned before, the latent heat capacity \(c^\text{l}\) is the quantity of main interest since it is correlated to the phase transformations. In order to determine \(c^\text{l}\), baseline correction is necessary. In [43], several approaches are discussed. For aluminium alloys, it has been suggested to measure the specific heat capacity of pure aluminium \(c_\text{r}\) and to subtract this value from the heat capacity of the sample \(c_\text{s}\) [33]. In pure aluminium, no significant endo- or exothermic reactions occur except the melting reaction, i.e. \(c_\text{r} = c_\text{r}^\text{s}\), cf. (4). Considering measurement errors, the specific heat capacity of pure aluminium and the sample are given by (cf. (7))
with the vectors of phase fractions of the pure aluminium and the sample \({\textbf{x}}_\text{r}\) and \({\textbf{x}}_\text{s}\). Since, the alloy content of the measured sample is usually very low, it can be assumed that the sensible heat capacity of the reference and the sample \(c_\text{r}^\text{s}\) and \(c_\text{s}^\text{s}\) are identical. Furthermore, in an ideal measurement setup, the errors \(\varepsilon _\text{r}\) and \(\varepsilon _\text{s}\) are the same. Therefore, these assumptions can be summarised as follows:
If the assumptions (10) hold, the latent heat capacity of the sample follows as
Nevertheless, the resulting DSC curve \(c_\text{s}^\text{l}\) may still be bent due to a non-ideal measurement setup. The remaining deviation has to be compensated by subtracting a linear function [16, 44, 55] or a polynomial function [32]. Alternatively, a correction function or a spline can be subtracted without prior subtraction of a pure aluminium curve [56]. The utilised function has to be fitted to reaction-free zones of the DSC curve. While a linear function can be placed through individual points, a polynomial function or a spline of higher order necessarily requires at least two reaction-free zones. If this is not guaranteed, the correction may result in an additional error. The correction of the DSC curve is quite decisive for a qualitative and quantitative analysis of the precipitation kinetics [32, 33, 39, 45]. A problem, especially with heat-flux DSC devices, where the measurement is made with an empty crucible as a reference, is the different inertia of the crucibles during heating. This causes overshoot artefacts at the start of the measurement [54], referred to as transient response [43]. Therefore, a suitable start temperature is crucial in order to capture the early-state precipitates by the DSC measurement, see "Influence of measurement start temperature" section. Most DSC curves in the literature start at 25–100 °C and thus cut off the onset of precipitation of the clusters and/or GP-zones or do not provide a signal in a steady state within this range.
Sample preparation
In principle, DSC samples of many geometries can be used. However, for improved reproducibility, the sample geometry within a series of measurements should not differ greatly, because of the reasons shown in "Parasitic effects in DSC" section. While the sample geometry mainly influences the measurement signal, the sample preparation can directly influence the reactions in the material: Punching discs from thin-walled materials using a pierce die is commonly used for fast and easy sample preparation. However, the punching can introduce lattice defects and influence the precipitation kinetics of second phases, leading to strongly distorted results, as has been reported by Birol for 6xxx series alloys [53, 57] and will be shown in this work also for 2xxx and 7xxx alloy series. The lattice effects caused by deformation usually accelerate the precipitation behaviour [58, 59], but decrease the phase fractions [53]. Unfortunately, many studies do not provide details on exact sample preparation procedures.
Influence of heating rate
The heating rate influences the precipitation kinetics and leads to a shift in the peak temperature of the individual phases. The peak temperatures are typically shifted to higher temperatures at higher heating rates and vice versa at lower heating rates. Additionally, peak broadening and changes in peak shape and thermal lag are possible. The influence of heating rates on DSC curves of aluminium has already been investigated extensively in prior research [32, 45, 54, 60 and 61], and thus is not considered in this work.
Aim of this work
For a reliable qualitative and quantitative analysis of DSC curves, the negative impact of sample preparation and experimental conditions on the DSC signal should be minimised as far as reasonably possible. Thus, we investigate the effects of sample preparation methods (i.e. punching before and after SHT versus milling), experimental conditions (i.e. the influence of quenching media, storage before measurement, and DSC start temperature), and baseline correction methods on DSC measurements of wrought aluminium alloys of the 2xxx, 6xxx, and 7xxx series in as-quenched and artificially aged tempers (W and T6, respectively). Such a comprehensive study is currently lacking in the literature.
Experimental setup and sample preparation
EN AW-2024, EN AW-6082, and EN AW-7075 sheets with thicknesses of 2 mm, 1.5 mm, and 2 mm, respectively, were used as well as pure aluminium (99.98 % purity) cut from a cast billet. The chemical composition of the alloys, measured by optical emission spectroscopy using a Spectro Spectromaxx 6, is shown in Table 1.
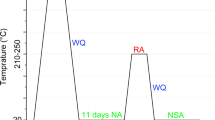
From each sheet, DSC samples were either punched with a piercing die (ø 5.3 mm) or milled using a CNC machine (ø 4.5 mm), see Fig. 3. Punching of samples was performed either before or after the SHT as well as after the artificial ageing using isopropanol as a lubricant. Punched samples were deburred using sandpaper. Milling of samples was only done from the sheet samples after T6 heat treatment. All samples were cleaned with isopropanol before being placed in the Al2O3 crucible. In order to investigate the influence of punching and milling on the DSC signal, DSC measurements were carried out under different heat treatment conditions, see Fig. 4.
SHT and AA were conducted according to the parameters given in Table 2 in a Nabertherm convection chamber furnace. Cooling from SHT was performed by quenching in water (room temperature). Cooling in air or quenching in liquid nitrogen was also performed to study the influence of the cooling rate. For determining the cooling rates during the cooling or quenching process, a type K thermocouple mounted on a DSC sample was used. The time at room temperature was kept as low as possible (< 1 min), except when the influence of natural ageing was studied. Samples undergoing subsequent T6 heat treatment after water cooling were stored at room temperature for approximately 5 min (time for sample and oven change). After artificial ageing, the samples were cooled at ambient air.
A DSC 204 F1 Phoenix device from Netzsch was used to record the DSC curves. To avoid parasitic effects, shown in "Parasitic effects in DSC" subsection, a correction (zeroline) measurement was carried out before the measurements with the same parameters as the subsequent DSC measurements [49]. The DSC sample was placed in the Al2O3 crucible and DSC chamber at room temperature (approximately 25 °C), the chamber was then subsequently cooled to the start temperature of −50 °C, unless otherwise stated, using liquid nitrogen. A temperature of 0 °C was reached after about 1.5 min, cooling to the critical temperature for precipitation [24, 62] of −40 °C took about 5 min. The start temperature was held for 10 min. For all measurements, the samples were heated to 600 °C with a heating rate of 10 K min−1. The measurement was always performed with a second empty reference Al2O3 crucible. The samples were placed in the crucible without touching the inner wall of the crucible. Each measurement was performed twice to ensure repeatability.
Results and discussion
Influence of measurement start temperature
As discussed in “Baseline correction of DSC curves”, at the start of the DSC measurement, switching from isothermal holding to linear heating leads to a transient response in the heat flow signal [54]. This can interfere with the baseline correction and with the analysis of low-temperature peaks, such as the peaks caused by the formation of early-stage precipitates (clusters and/or GP-zones), which can start already around \(\sim\)40 °C [63]. We used EN AW-6082-T6 to study the end of the transient response at different start temperatures of the measurement. EN AW-6082-T6 is suitable for this purpose, because no low-temperature formation peaks occur, as the material is already artificially aged.
The results are shown in Fig. 5. When the measurement is started at 25 °C, the end of the transient response is around 90 °C, which means that the early peaks in many alloys or tempers would be confounded (as will be shown in Sec. 3.4). When the measurement is started at 0 °C, the signal is stable at \(\sim\)40 °C, which would be sufficient to record the early peaks. However, for baseline correction, which will be shown below, a reaction-free zone of a width of at least \(\sim\)20 °C is desirable [32], which means that a lower start temperature should be chosen. Of the start temperatures tested, −50 °C fulfils this requirement. This is in agreement with the findings of Chang and Banhart, who started their DSC curves for the investigation of clustering in an Al-Mg-Si alloy at −50 °C [63]. Therefore, we used this start temperature for all further measurements.
Baseline correction
As shown in "Baseline correction of DSC curves" section, a baseline correction can be performed by subtracting a pure aluminium curve and compensating for the remaining curvature by subtracting a polynomial fitted to the reaction-free zones of the DSC signal. Here, we investigate whether subtraction of pure aluminium is necessary, subtracting a polynomial alone is permissible, or the combined approach is advisable. We also discuss possibilities for baseline correction in the absence of clear reaction-free zones.
In Fig. 6a, the baseline correction of an EN AW-6082-W DSC curve by subtraction of only a pure aluminium curve is shown. It can be seen that the curvature of the pure aluminium curve is similar to that of the alloy, particularly in the reaction-free zones in the beginning and end of the alloy curve (\(\sim\)10–30 °C and \(\sim\)510–550 °C, respectively). Overall, the baseline correction appears acceptable, but some offset remains in the reaction-free zone at high-temperature.
In Fig. 6b, an approach using only a polynomial subtraction is shown. The reaction-free zones, to which the polynomial of third order is fitted, are highlighted in yellow. In addition to the correction of the curvature, no offset in the high-temperature reaction-free zone remains.
The combined approach is shown in Fig. 6c. After the subtraction of pure aluminium, only a small curvature remains. Thus, the polynomial fitted to the reaction-free zones appears close to a linear function in the shown temperature region and serves well to remove the remaining offset. The resulting curve is almost identical to the curve shown in Fig. 6b.
In view of the above results, it seems justified to dispense with the measurement and curve subtraction of pure aluminium. However, the results of fitting a polynomial are highly dependent on the supporting points chosen and first subtracting pure aluminium may be more robust. Furthermore, not all alloys show at least two clear reaction-free zones for fitting the polynomial. An example is EN AW-2024-W, see Fig. 7a. Here, subtraction of a pure aluminium curve can serve to remove the curvature, and the remaining offset is removed using a linear function fitted to the curve before the onset of melting. In the light of the results shown in Fig. 6c, this procedure can be expected to yield an acceptable baseline correction.
In Fig. 7b, baseline correction for EN AW-7075-W is shown. Here, some curvature remains after subtraction of pure aluminium, and fitting a polynomial appears more suitable than a linear function.
In summary, for baseline correction, we suggest subtracting a pure aluminium curve and, subsequently, a polynomial fitted to the reaction-free zones. When two clear reaction-free zones are not present, a linear function can be used instead of the polynomial.
Influence of quenching media and natural ageing
For measurements in the W-temper, it is necessary to avoid precipitation during cooling and sample transfer (natural ageing) to start with a true SSSS.
To prevent precipitation during quenching, the critical cooling rate must be reached [50]. For EN AW-6082, it has been found to be 15 K s−1 [64].
For a given geometry, the cooling rate depends on the quenching media (e.g. air, water and liquid nitrogen) and the chemical composition of the sample [52]. Figure 8a shows the cooling rates achieved for EN AW-6082 by using different quenching media. It is well known that air cooling does not achieve the critical cooling rates required for most Al wrought alloys and this is also the case here. In contrast, both water and liquid nitrogen easily fulfil the 15 K s−1 requirement. The cooling rate during quenching in water is much higher than in liquid nitrogen, which may be explained by the less pronounced Leidenfrost effect [65].
Compared to water or air, liquid nitrogen as a quenching medium has the advantage that the sample can be directly brought to −196 °C, where no further precipitation occurs [24, 62]. Therefore, a time delay between the SHT and the DSC measurement is not an issue. When the measurement is then started at −50 °C, a true W-temper can be assumed.
Influence of quenching media and storage at room temperature (natural ageing) on the DSC signal of EN AW-6082: (a) Quenching experiments of DSC samples of EN AW-6082 (sheet thickness of 1.5 mm) in different media with achieved cooling rates and (b) baseline-corrected DSC heating curves of samples quenched after SHT with different cooling media (liquid N\(_2\) or H\(_2\)O) and different time delays (1 or 5 min)
Figure 8b shows three DSC curves of EN AW-6082 quenched in water or liquid nitrogen. For the water-quenched samples, the delay before the DSC measurements was 1 or 5 min. For liquid nitrogen, the delay is irrelevant. The three curves appear very similar for peak A, related to the formation of clusters and/or GP-zones, especially for the water-quenched samples. The slight difference for peak A in the sample quenched with liquid nitrogen could be related to the observations of Liu et al. [17], who showed that clustering can also occur during cooling by using positron lifetime experiments. The solute atoms that already formed clusters during cooling are thus no longer available for cluster formation during heating in the DSC.
As the precipitation sequence progresses, a difference in the endothermic peak B (dissolution of clusters and/or GP-zones) can be seen between the three curves, see Fig. 8b. While the area ratio of formation and dissolution peaks A and B is near unity for samples quenched in liquid nitrogen (ratio of 0.86, see also shaded area in Fig. 8b), the ratio is lower for samples quenched in water, especially after a delay of 5 min, see Table 3. A ratio \(<1\) means that there is more dissolution than formation, which is impossible if starting from a true SSSS. Therefore, it can be concluded that some formation of early-stage precipitates has taken place in all samples, but less for the samples quenched in liquid nitrogen.
Overall, the differences between the three curves over the whole temperature range are minor and both water and liquid nitrogen can be considered as a suitable quenching media, unless early-stage precipitates are of particular interest. Furthermore, small delays between quenching and measurement are acceptable. Nevertheless, the delay should be kept as low as possible (e.g. 1 min). In light of the higher industrial relevance of water quenching, we used water for the following measurements.
Influence of sample preparation
The DSC samples were either punched or milled as shown in "Experimental setup and sample preparation" section (Fig 3.). The effect of sample preparation on the baseline-corrected DSC curves of alloys EN AW-6082, EN AW-2024 and EN AW-7075 in W-temper or T6 are shown in Figs. 9–11.
In Fig. 9a, two DSC heating curves of the EN AW-6082 alloy after SHT are shown, where samples were punched before and after the SHT, respectively. Punching the samples after heat treatment leads to a decrease in precipitation density of clusters and/or GP-zones (peak A), also observed by Birol et al. [53]. The decrease is likely due to the annihilation of quenched-in vacancies by dislocations introduced by deformation [66]. Subsequently, punching also appears to accelerate the formation of \(\beta ''\) (peak C), a well-known effect of pre-deformation shown by different authors [67,68,69]. The formation of \(\beta '\) (peak C’) is suppressed. This effect also leads to smaller peak areas during the dissolution of the \(\beta '\)-phase (peak D) but earlier formation of the \(\beta\)-phase (peak E). Additionally, a slight shift of the peak temperatures occurs. In addition to changes to the precipitation kinetics by deformation, further parasitic effects are conceivable. When the sample is punched after SHT, its side surface has a shiny surface, causing other thermal radiation effects. Deburring with sandpaper has a similar effect. The distinction between these effects could be part of future work.
In T6 temper (see Fig. 9b), the DSC curves of the sample punched before SHT and punched or milled after the heat treatments (SHT + AA) show good agreement in the peak temperatures but still some differences in the peak areas. The peak areas are reduced for the samples punched after the heat treatments, while the curves of the samples punched before SHT or milled from T6 are very similar.
Similar observations can be made for EN AW-2024 in W-temper. Figure 10a shows that punching the sample after SHT results in a strong influence on the DSC curves and leads to a reduction of peak G, which represents the precipitation of clusters and/or GP-zones [30]. Regarding the peak temperatures, an even greater shift can be observed compared to EN AW-6082-W. The reduction of peak G could be explained by a reduced formation of clusters and/or GP-zones due to the introduced dislocations, which reduce the number of vacancies [70]. Consequently, peak H, which is thought to be related to the dissolution of the previously formed clusters and/or GP-zones, is also reduced. Additionally, a clear shift in the peak temperature and shape of peaks I and I\('\), related to the formation of the S-phase, can be seen. Peak I starts approximately 20 °C earlier for samples punched after SHT. Applying pre-strain can also lead to an accelerated precipitation behaviour as shown by several authors [70,71,72], which could explain this observation. According to Wang et al. [30], the I/I\('\) double peak is due to the formation of the S-phase types 1 and 2. For DSC samples punched after SHT, the second peak I\('\) does not occur. This could be explained by the increased dislocation density since dislocations serve as heterogeneous nucleation sites and accelerate the formation of S-phase type 2 [30]. Overall, the S-phase precipitation volume (peak area of I/I\('\)) is significantly increased by punching prior to SHT.
Figure 10b shows that the peak G can not be observed in the T6-state, and the peak areas of peaks H and I are significantly reduced compared to W-temper. Moreover, peaks H and I are less pronounced for the sample punched from T6 compared to the sample punched before SHT and artificial ageing. The curve for the sample milled from T6 is essentially the same as the curve of the sample punched before heat treatment. Peak J represents the dissolution of the S-phase and appears to be not affected by sample preparation. The same can be observed for peak K, which is related to the dissolution of the high-temperature phase (e.g. \(\theta\) or S eutectic [23]).
For EN AW-7075 in W-temper, punching after SHT strongly affects the areas of peaks L, N and N\('\), while only minor shifts of the peak temperatures can be shown, see Fig. 11a. The reduction of peak L, related to clusters and GP-zones [10, 73], may be due to the annihilation of quenched-in vacancies by introduced dislocations [70]. The slight shift to a lower temperature for the sample punched after SHT may be due to increased diffusivity through dislocations and was also observed by Panigrahi et al. on cryo-rolled 7075 material [74] and Aster et al. on Al-Zn-Mg-Cu cross-over alloys [75]. Accelerated clustering would also have the effect that fewer solutes are available for further clustering when the sample is heated in the DSC, which could explain why peak L is significantly smaller for the sample punched after SHT. Compared to EN AW-6082 and EN AW-2024, the peaks N and N\('\) are significantly reduced for the sample punched before SHT compared to the sample punched after SHT. The increase of peaks N and N\('\) indicates an increase of precipitation kinetics of the \(\eta '\)- and \(\eta\)-phases due to punching the samples [55, 76].
In Fig. 11b, the DSC curves of EN AW-7075-T6 show almost no influence of sample preparation. Only peak M, likely related to the dissolution of GP-zones and fine \(\eta '\)-phases [55, 77], is slightly reduced in height for samples punched from T6. Besides peak M, the curves are almost congruent. The lower influence of punching the sample after SHT and artificial ageing may be related to the high strength of EN AW-7075-T6, which limits plastic deformation during punching.
Conclusions
For qualitative and quantitative interpretation of DSC curves, representative and reproducible measurements as well as low-bias baseline correction are desirable. To this end, we studied the influences of experimental conditions, baseline correction, and sample preparation. The following conclusions can be drawn:
-
The start temperature of the measurement is crucial for low-bias baseline correction and interpretation of low-temperature peaks related to early-stage precipitates. Starting the measurement at a low-temperature (−50 °C) is advised. Pure aluminium should be used as reference material and additional correction of the remaining curvature can be achieved using a linear or polynomial correction function fitted to reaction-free zones.
-
The choice of quenching media (water or liquid nitrogen) has only a small influence on the curves. The same can be said for short durations of room temperature storage (i.e. 1 or 5 min) before measurement. However, if early-stage precipitates are of particular interest, quenching in liquid nitrogen may be more suitable, as natural ageing before measurement can be avoided.
-
Preparation of DSC samples with a piercing die can have a strong influence on the precipitation kinetics: A major influence on DSC peak temperatures, shapes, and areas was found for 2xxx, 6xxx, and 7xxx series alloys. This influence can be avoided by punching before solution heat treatment (SHT), so that the sample can undergo recovery. If punching before SHT is not possible, for example, when DSC samples are to be taken from semi-finished goods or from components, milling is a good alternative to prevent plastic deformation of the samples, which is thought to be the main cause of the influence on precipitation kinetics.
In order to obtain high-quality DSC curves, these points should be taken into account to significantly reduce measurement or interpretation errors.
References
Ostermann F. Anwendungstechnologie aluminium. Cham: Springer-Verlag; 2015. https://doi.org/10.1007/978-3-662-43807-7.
Friedrich HE. Leichtbau in der Fahrzeugtechnik. Cham: Springer-Verlag; 2017. https://doi.org/10.1007/978-3-658-12295-9.
Zhou B, Liu B, Zhang S. The advancement of 7xxx series aluminum alloys for aircraft structures: a review. Metals. 2021;11(5):718. https://doi.org/10.3390/met11050718.
Dursun T, Soutis C. Recent developments in advanced aircraft aluminium alloys. Mater Design (1980-2015). 2014;56:862–71. https://doi.org/10.1016/j.matdes.2013.12.002.
Schiffl A, Kühlein W. Hochfeste Crashlegierungen aus dem Haus Hammerer Aluminium Industries: von der Idee zur Umsetzung. DOI. 2014;10(2.1):4397–7601. https://doi.org/10.13140/2.1.4397.7601.
Hirsch J. Recent development in aluminium for automotive applications. Trans Nonferrous Metals Soc China. 2014;24(7):1995–2002. https://doi.org/10.1016/S1003-6326(14)63305-7.
Ismail A, Mohamed M. Review on sheet metal forming process of aluminium alloys, in: Proceedings of the 17th International Conference on Applied Mechanics and Mechanical Engineering, Cairo, Egypt, 2016:19–21. https://doi.org/10.21608/amme.2016.35204.
Prillhofer R, Rank G, Berneder J, Antrekowitsch H, Uggowitzer PJ, Pogatscher S. Property criteria for automotive Al–Mg–Si sheet alloys. Materials. 2014;7(7):5047–68. https://doi.org/10.3390/ma7075047.
Polmear I, Aluminium Alloys -A. Century of Age Hardening, in: Materials forum, 2004:28;13.
Watzl G, Grünsteidl C, Arnoldt A, Nietsch JA, Österreicher JA. In situ laser-ultrasonic monitoring of elastic parameters during natural aging in an Al–Zn–Mg–Cu alloy (AA7075) sheet. Materialia. 2022;26:101600. https://doi.org/10.1016/j.mtla.2022.101600.
Geng Y, Zhang D, Zhang J, Zhuang L. Early-stage clustering and precipitation behavior in the age-hardened Al–Mg–Zn (–Cu) alloys. Mater Sci Eng A. 2022;856:144015. https://doi.org/10.1016/j.msea.2022.144015.
Werinos M, Antrekowitsch H, Ebner T, Prillhofer R, Uggowitzer PJ, Pogatscher S. Hardening of Al–Mg–Si alloys: effect of trace elements and prolonged natural aging. Mater Design. 2016;107:257–68. https://doi.org/10.1016/j.matdes.2016.06.014.
Marceau RK, Sha G, Ferragut R, Dupasquier A, Ringer SP. Solute clustering in Al–Cu–Mg alloys during the early stages of elevated temperature ageing. Acta Mater. 2010;58(15):4923–39. https://doi.org/10.1016/j.actamat.2010.05.020.
Pogatscher S, Antrekowitsch H, Leitner H, Ebner T, Uggowitzer PJ. Mechanisms controlling the artificial aging of Al–Mg–Si Alloys. Acta Mater. 2011;59(9):3352–63. https://doi.org/10.1016/j.actamat.2011.02.010.
Ratchev P, Verlinden B, De Smet P, Van Houtte P. Precipitation hardening of an Al–4.2 wt% Mg–0.6 wt% Cu alloy. Acta Mater. 1998;46(10):3523–33. https://doi.org/10.1016/S1359-6454(98)00033-0.
Österreicher J, Nebeling D, Grabner F, Cerny A, Zickler G, Eriksson J, Wikström G, Suppan W, Schlögl C, Secondary ageing and formability of an Al-Cu-Mg alloy. in W and under-aged tempers. Mater Design. 2024;2023: 111634. https://doi.org/10.1016/j.matdes.2023.111634.
Liu M, Čížek J, Chang CS, Banhart J. Early stages of solute clustering in an Al–Mg–Si alloy. Acta Mater. 2015;91:355–64. https://doi.org/10.1016/j.actamat.2015.02.019.
Banhart J, Chang CST, Liang Z, Wanderka N, Lay MD, Hill AJ. Natural aging in Al–Mg–Si alloys: a process of unexpected complexity. Adv Eng Mater. 2010;12(7):559–71. https://doi.org/10.1002/adem.201000041.
Lervik A, Thronsen E, Friis J, Marioara CD, Wenner S, Bendo A, Matsuda K, Holmestad R, Andersen SJ. Atomic structure of solute clusters in Al–Zn–Mg alloys. Acta Mater. 2021;205:116574. https://doi.org/10.1016/j.actamat.2020.116574.
Engler O, Marioara C, Aruga Y, Kozuka M, Myhr O. Effect of natural ageing or pre-ageing on the evolution of precipitate structure and strength during age hardening of Al–Mg–Si alloy AA 6016. Mater Sci Eng A. 2019;759:520–9. https://doi.org/10.1016/j.msea.2019.05.073.
Dorin T, Ramajayam M, Babaniaris S, Jiang L, Langan T. Precipitation sequence in Al–Mg–Si–Sc–Zr alloys during isochronal aging. Materialia. 2019;8:100437. https://doi.org/10.1016/j.mtla.2019.100437.
Berg L, Gjønnes J, Hansen V, Li X, Knutson-Wedel M, Schryvers D, Wallenberg L. GP-zones in Al–Zn–Mg alloys and their role in artificial aging. Acta Mater. 2001;49(17):3443–51. https://doi.org/10.1016/S1359-6454(01)00251-8.
Ghosh K. Calorimetric studies of 2024 Al–Cu–Mg and 2014 Al–Cu–Mg–Si alloys of various tempers. J Therm Anal Calorim. 2019;2024(136):447–59. https://doi.org/10.1007/s10973-018-7702-0.
Røyset J, Stene T, Sæter J. A, Reiso O. The effect of intermediate storage temperature and time on the age hardening response of Al–Mg–Si alloys, in: Materials Science Forum, Vol. 519, Trans Tech Publ, 2006:239–244. https://doi.org/10.4028/www.scientific.net/MSF.519-521.239.
Esmaeili S, Wang X, Lloyd D, Poole W. On the precipitation-hardening behavior of the Al–Mg–Si–Cu alloy AA6111. Metall Mater Trans A. 2003;34:751–63. https://doi.org/10.1007/s11661-003-1003-2.
Liu C, Malladi SK, Xu Q, Chen J, Tichelaar FD, Zhuge X, Zandbergen HW. In-situ STEM imaging of growth and phase change of individual CuAlX precipitates in Al alloy. Sci Rep. 2017;7(1):2184. https://doi.org/10.1038/s41598-017-02081-9.
Cao C, Zhang D, He Z, Zhuang L, Zhang J. Enhanced and accelerated age hardening response of Al-5.2 Mg–0.45 Cu (wt%) alloy with Zn addition. Mater Sci Eng A. 2016;666:34–42. https://doi.org/10.1016/j.msea.2016.04.022.
Stemper L, Tunes MA, Dumitraschkewitz P, Mendez-Martin F, Tosone R, Marchand D, Curtin WA, Uggowitzer PJ, Pogatscher S. Giant hardening response in Al–Mg–Zn (Cu) alloys. Acta Mater. 2021;206:116617. https://doi.org/10.1016/j.actamat.2020.116617.
Sha G, Marceau R, Gao X, Muddle B, Ringer S. Nanostructure of aluminium alloy 2024: segregation, clustering and precipitation processes. Acta Mater. 2011;59(4):1659–70. https://doi.org/10.1016/j.actamat.2010.11.033.
Wang S, Starink M. Two types of S phase precipitates in Al–Cu–Mg alloys. Acta Mater. 2007;55(3):933–41. https://doi.org/10.1016/j.actamat.2006.09.015.
Andersen S, Zandbergen H, Jansen J, Træholt C, Tundal U, Reiso O. The crystal structure of the \(\beta\)" phase in Al–Mg–Si alloys. Acta Mater. 1998;46(9):3283–98. https://doi.org/10.1016/S1359-6454(97)00493-X.
Osten J, Milkereit B, Schick C, Kessler O. Dissolution and precipitation behaviour during continuous heating of Al–Mg–Si alloys in a wide range of heating rates. Materials. 2015;8(5):2830–48. https://doi.org/10.3390/ma8052830.
Milkereit B, Starink M. Quench sensitivity of Al–Mg–Si alloys: a model for linear cooling and strengthening. Mater Design. 2015;76:117–29. https://doi.org/10.1016/j.matdes.2015.03.055.
Edwards G, Stiller K, Dunlop G, Couper M. The precipitation sequence in Al–Mg–Si alloys. Acta Mater. 1998;46(11):3893–904. https://doi.org/10.1016/S1359-6454(98)00059-7.
Sha G, Cerezo A. Early-stage precipitation in Al–Zn–Mg–Cu alloy (7050). Acta Mater. 2004;52(15):4503–16. https://doi.org/10.1016/j.actamat.2004.06.025.
Österreicher JA, Kirov G, Gerstl SS, Mukeli E, Grabner F, Kumar M. Stabilization of 7xxx aluminium alloys. J Alloy Compd. 2018;740:167–73. https://doi.org/10.1016/j.jallcom.2018.01.003.
Ma K, Wen H, Hu T, Topping TD, Isheim D, Seidman DN, Lavernia EJ, Schoenung JM. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2014;62:141–55. https://doi.org/10.1016/j.actamat.2013.09.042.
Inoue H, Sato T, Kojima Y, Takahashi T. The temperature limit for GP zone formation in an Al–Zn–Mg alloy. Metall Mater Trans A. 1981;12:1429–34. https://doi.org/10.1007/BF02643687.
Jiang X, Tafto J, Noble B, Holme B, Waterloo G. Differential scanning calorimetry and electron diffraction investigation on low-temperature aging in Al–Zn–Mg alloys. Metall Mater Trans A. 2000;31:339–48. https://doi.org/10.1007/s11661-000-0269-x.
Quick CR, Dumitraschkewitz P, Schawe JEK, Pogatscher S. Fast differential scanning calorimetry to mimic additive manufacturing processing: specific heat capacity analysis of aluminium alloys. J Therm Anal Calorim. 2023;148(3):651–62. https://doi.org/10.1007/s10973-022-11824-4.
Moran M, Shapiro H, Boettner D, Bailey M. Fundamentals of engineering thermodynamics. New York: Wiley, 2014: https://books.google.at/books?id=uxObAwAAQBAJ
Netzsch, Functional principle of a heat-flux dsc, https://analyzing-testing.netzsch.com/en/landingpages/principle-of-a-heat-flux-dsc, Last accessed on 2023-10-10.
Höhne GWH, Hemminger WF, Flammersheim HJ. Differential scanning calorimetry. Cham: Springer 2003.
Arnoldt AR, Schiffl A, Höppel HW, Österreicher JA. Influence of different homogenization heat treatments on the microstructure and hot flow stress of the aluminum alloy AA6082. Mater Charact. 2022;191:112129. https://doi.org/10.1016/j.matchar.2022.112129.
Fröck H, Rowolt C, Milkereit B, Reich M, Kowalski W, Stark A, Kessler O. In situ high-energy X-ray diffraction of precipitation and dissolution reactions during heating of Al alloys. J Mater Sci. 2021;56(35):19697–708. https://doi.org/10.1007/s10853-021-06548-z.
Pishchur D, Drebushchak V. Recommendations on DSC calibration How to escape the transformation of a random error into the systematic error. J Therm Anal Calorim. 2016;124:951–8. https://doi.org/10.1007/s10973-015-5186-8.
Della Gatta G, Richardson M, Sarge S, Stølen S. Standards, calibration, and guidelines in microcalorimetry. Part 2. Calibration standards for differential scanning calorimetry* (IUPAC Technical Report), Pure and Applied Chemistry - PURE APPL CHEM 2006:78;1455–1476. https://doi.org/10.1351/pac200678071455.
Schubnell M. Temperature and heat flow calibration of A DSC-instrument in the temperature range between −100 and 160°C. J Therm Anal Calorim. 2000;61:91–8. https://doi.org/10.1023/A:1010156407261.
Wilthan B. Bestimmung der spezifischen Wärmekapazität von Stoffen mittels dynamischer Differenzkalorimetrie., Ph.D. thesis 2002.
Kessler O, Milkereit B, Schick C. Quench sensitivity and continuous cooling precipitation diagrams. Encycl. of Alumin. Alloys, Two-Volume Set 2019:2189–2217. https://doi.org/10.1201/9781351045636-140000288.
Kočí V, Fořt J, Maděra J, Scheinherrová L, Trník A, Černý R. Correction of errors in DSC measurements using detailed modeling of thermal phenomena in calorimeter-sample system. IEEE Trans Instrum Meas. 2020;69(10):8178–86. https://doi.org/10.1109/TIM.2020.2987454.
Milkereit B, Kessler O, Schick C. Recording of continuous cooling precipitation diagrams of aluminium alloys. Thermochim Acta. 2009;492:73–8. https://doi.org/10.1016/j.tca.2009.01.027.
Birol Y. DSC analysis of the precipitation reactions in the alloy AA6082: effect of sample preparation. J Therm Anal Calorim. 2006;83(1):219–22. https://doi.org/10.1007/s10973-005-6950-y.
Milkereit B. Kontinuierliche Zeit-Temperatur-Ausscheidungs-Diagramme von Al–Mg–Si-Legierungen, Ph.D. thesis, Rostock, Univ., Diss., 2011:2011. https://doi.org/10.2370/9783832299934.
Österreicher JA, Grabner F, Tunes MA, Coradini DS, Pogatscher S, Schlögl CM. Two step-ageing of 7xxx series alloys with an intermediate warm-forming step. J Market Res. 2021;12:1508–15. https://doi.org/10.1016/j.jmrt.2021.03.062.
Tutorial Guide–DSC Data Analysis in Origin, https://bif.wisc.edu/wp-content/uploads/sites/389/2017/11/DSC_Data_Analysis_in_Origin.pdf, Version 7.0 (2004).
Birol Y. The effect of sample preparation on the DSC analysis of 6061 alloy. J Mater Sci. 2005;40(24):6357–61.
Gazizov M, Kaibyshev R. Effect of pre-straining on the aging behavior and mechanical properties of an Al–Cu–Mg–Ag alloy. Mater Sci Eng A. 2015;625:119–30. https://doi.org/10.1016/j.msea.2014.11.094.
Guía-Tello J, Garay-Reyes C, Rodríguez-Cabriales G, Medrano-Prieto H, Ruiz-Esparza-Rodríguez M, Hernández LG, Mendoza-Duarte J, García-Aguirre K, Estrada-Guel I, González S, Effect of plastic deformation on the precipitation sequence of, et al. aluminum alloy. J Mater Sci. 2024;2022:1–14. https://doi.org/10.1007/s10853-021-06689-1.
Kuijpers N, Kool W, van der Zwaag S. DSC study on Mg–Si phases in as cast AA6xxx, in: Materials Science Forum, Vol. 396, Trans Tech Publ, 2002:675–680. https://doi.org/10.4028/www.scientific.net/MSF.396-402.675.
Kessler O, Milkereit B, Schick C. Quench sensitivity and continuous cooling precipitation diagrams, in: Encyclopedia of Aluminum and Its Alloys, Two-Volume Set (Print), CRC Press, Boca Raton, 2018;2189–2217
Dumitraschkewitz P, Uggowitzer PJ, Gerstl SS, Löffler JF, Pogatscher S. Size-dependent diffusion controls natural aging in aluminium alloys. Nat Commun. 2019;10(1):4746. https://doi.org/10.1038/s41467-019-12762-w.
Chang C, Banhart J. Low-temperature differential scanning calorimetry of an Al–Mg–Si alloy. Metall Mater Trans A. 2011;42:1960–4. https://doi.org/10.1007/s11661-010-0596-5.
Shang B, Yin Z, Wang G, Liu B, Huang Z. Investigation of quench sensitivity and transformation kinetics during isothermal treatment in 6082 aluminum alloy. Mater Design. 2011;32(7):3818–22. https://doi.org/10.1016/j.matdes.2011.03.016.
Gottfried B, Lee C, Bell K. The Leidenfrost phenomenon: film boiling of liquid droplets on a flat plate. Int J Heat Mass Transf. 1966;9(11):1167–88. https://doi.org/10.1016/0017-9310(66)90112-8.
Fischer FD, Svoboda J, Appel F, Kozeschnik E. Modeling of excess vacancy annihilation at different types of sinks. Acta Mater. 2011;59(9):3463–72. https://doi.org/10.1016/j.actamat.2011.02.020.
Kolar M, Pedersen KO, Gulbrandsen-Dahl S, Marthinsen K. Combined effect of deformation and artificial aging on mechanical properties of Al–Mg–Si alloy. Trans Nonferrous Metals Soc China. 2012;22(8):1824–30. https://doi.org/10.1016/S1003-6326(11)61393-9.
Jia Z-H, Ding L-P, Weng Y-Y, Zhang W, Qing L. Effects of high temperature pre-straining on natural aging and bake hardening response of Al–Mg–Si alloys. Trans Nonferrous Metals Soc China. 2016;26(4):924–9. https://doi.org/10.1016/S1003-6326(16)64188-2.
Österreicher JA. Combined cyclic deformation and artificial ageing of an Al–Mg–Si alloy. Mater Lett: X. 2021;10:100072. https://doi.org/10.1016/j.mlblux.2021.100072.
Naimi A, Yousfi H, Trari M. Influence of cold rolling degree and ageing treatments on the precipitation hardening of 2024 and 7075 alloys. Mech Time-Depend Mater. 2013;17:285–96. https://doi.org/10.1007/s11043-012-9182-0.
Ying P, Liu Z, Bai S, Liu M, Lin L, Xia P, Xia L. Effects of pre-strain on Cu–Mg co-clustering and mechanical behavior in a naturally aged Al–Cu–Mg alloy. Mater Sci Eng A. 2017;704:18–24. https://doi.org/10.1016/j.msea.2017.06.097.
Zuiko I, Kaibyshev R. Effect of plastic deformation on the ageing behaviour of an Al–Cu–Mg alloy with a high Cu/Mg ratio. Mater Sci Eng A. 2018;737:401–12. https://doi.org/10.1016/j.msea.2018.09.017.
Viana F, Pinto A, Santos H, Lopes A. Retrogression and re-ageing of 7075 aluminium alloy: microstructural characterization. J Mater Process Technol. 1999;92:54–9. https://doi.org/10.1016/S0924-0136(99)00219-8.
Panigrahi SK, Jayaganthan R. Effect of ageing on microstructure and mechanical properties of bulk, cryorolled, and room temperature rolled Al 7075 alloy. J Alloy Compd. 2011;509(40):9609–16. https://doi.org/10.1016/j.jallcom.2011.07.028.
Aster P, Dumitraschkewitz P, Uggowitzer PJ, Schmid F, Falkinger G, Strobel K, Kutlesa P, Tkadletz M, Pogatscher S. Strain-induced clustering in Al alloys. Available at SSRN. 4526728. https://doi.org/10.2139/ssrn.4526728.
Jung S-H, Lee J, Kawasaki M. Effects of pre-strain on the aging behavior of al 7075 alloy for hot-stamping capability. Metals. 2018;8(2):137. https://doi.org/10.3390/met8020137.
Tang J-G, Hui C, Zhang X-M, Liu S-D, Liu W-J, Ouyang H, Li H-P. Influence of quench-induced precipitation on aging behavior of Al–Zn–Mg–Cu alloy. Trans Nonferrous Metals Soc China. 2012;22(6):1255–63. https://doi.org/10.1016/S1003-6326(11)61313-7.
Funding
Open access funding provided by AIT Austrian Institute of Technology GmbH. This work received financial support by the State of Upper Austria (project Data-T-Rex, Grant no. Wi-2021-305676/13-Au).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Aurel Arnoldt and Lukas Grohmann. The first draft of the manuscript was written by Aurel Arnoldt and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Declarations
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arnoldt, A.R., Grohmann, L., Strommer, S. et al. Differential scanning calorimetry of age-hardenable aluminium alloys: effects of sample preparation, experimental conditions, and baseline correction. J Therm Anal Calorim 149, 4425–4439 (2024). https://doi.org/10.1007/s10973-024-13019-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-024-13019-5