Abstract
The paper describes the preparation of polyamide 6 with reduced flammability by anionic in situ polymerization of ε-caprolactam in the presence of flame retardants, a method not described in the literature. Selected inorganic and brominated organic flame retardants were characterized by thermogravimetric analysis. Polymerization tests were used to test the compatibility of flame retardants with the components of the initiation system of anionic polymerization. Based on their results, polyamide plates for the preparation of test specimens were prepared by the method of polymerization casting in a mold. The content of water-extractable portions did not exceed 3%, i.e., the conversion of monomer into polymer took place to a high degree. The presence of flame retardants both inorganic and brominated organic in the polymer matrix did not affect the thermal stability, the content of the crystalline phase and also the mechanical properties. Samples containing brominated organic flame retarders were classified as V-0 according to the UL94 methodology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Linear aliphatic polyamides (PAs) are among the most important polymers, both for some exceptional properties and for their annual global production volume of over 7 Mt. The amide bond –CO–NH– is regularly repeated in the polyamide macromolecules, and the different types of aliphatic polyamides differ in the number of methylene groups between the amide groups and are identified by a name or symbol (PA or NY) and a numerical value corresponding to the number of carbon atoms in the monomer(s) used to synthesize the polyamide. They are produced by polycondensation of diamines and dicarboxylic acids (e.g., PA46, PA66 and PA612), polycondensation of amino acids (PA11) or polymerization of cyclic monomers—lactams (PA6, PA12) [1].
Polyamides are predominantly used in the fiber industry, but their application as structural materials in various industries is growing rapidly. Polyamides are characterized by excellent mechanical properties, high modulus and hardness, relatively high toughness and abrasion resistance. With the addition of microfillers (e.g., glass fibers, graphite, limestone), filled polyamides are commonly produced, the resulting properties of which depend on the type and concentration (10–40%) of the filler. Its use may be limited by its low resistance to burning. Reduction in flammability is achieved by incorporating flame retardants into the polymeric material.
Polyamide 6 (PA6) filled with flame retardants is prepared by melt blending, which is produced by hydrolytic polymerization of ε-caprolactam (CL). The way of incorporating flame retardant into PA6 is a critical point of the whole preparation, since the flame retardant must not interfere with the blending process of the polyamide and also the formation of the final product, i.e., reduce the polymerization degree and degrade the polymer. The main flame retardants used are magnesium hydroxide, melamine cyanurate/phosphate, polybrominated aromatics, antimony oxide, ammonium polyphosphate/sulfamate, etc. [2,3,4,5,6,7]. Another possibility is the use of hydrolytic polymerization of CL in the presence of a flame retardant as described in publications [8, 9]. During this polymerization (polycondensation), these compounds cause an imbalance of functional groups and thus a significant decrease in the molar mass of the resulting PA6.
Technologies using high rates of anionic polymerization of CL, which allow to combine polymerization and product forming into one technological unit, so-called polymer casting (monomer casting) in static or rotating molds, reaction injection molding (RIM) and reaction extrusion, have a special position [1]. These technologies enable the production of large products of various shapes, such as plates, tubes, gears and fuel tanks. There is no publicly available literature in the field of reduced flammability PA6 prepared by anionic polymerization of CL. This is due to the known sensitivity of the components of the anionic polymerization initiation system to the presence of a number of compounds [10], not excluding flame retardants.
The aim of the present work is the preparation and characterization of polyamide 6 with reduced flammability, i.e., by anionic in situ intercalation polymerization of CL containing flame retardant.
Experimental
Materials
ε-Caprolactam (CL) (DSM) was used without further purification and stored in a desiccator over P2O5, water content 65 ppm—determined by Karl Fischer coulometric titration, and ε-caprolactam magnesium bromide (CLMgBr) concentrate in CL (Bruggolen® C1). The content of Mg2+ was 1.02 mol kg−1 (determined by chelatometric titration). Sodium ε-caprolactamate (CLNa) in CL (1.29 mol Na+.kg−1) in the form of flakes (Bruggolen® C10) and hexamethylene diisocyanate blocked with CL (HDCL) (Bruggolen® C20) containing 4 mol carbamoyl group per kg were supplied by Brüggemann. Dilactamate initiator—sodium di(ε-caprolactamato)-bis(2-methoxyethoxo) aluminate (DL), molar mass 424.4 g mol−1, solution in toluene 82%, was supplied by Katchem CZ. N,N-isophthaloyl-bis-ε-caprolactam (IPBCL) was prepared as described in [11]. All chemicals except CL were stored under the protective argon atmosphere.
Flame retardants
Magnesium carbonate (Lachema Brno, CZ), magnesium hydroxide (Sigma-Aldrich), aluminum hydroxide (Lachema Brno, CZ) and antimony trioxide 99% (Merck) were dried in vacuum at 80 °C/50 Pa for 8 h prior to use.
Tris(tribromoneopenthyl)phosphate (FR-370, bromine content 70%), 1,1 ‘-(isopropyliden)bis[3,5-dibromo-4-(2,3-dibromo-2-methylpropoxy)benzen (SC BDMP 66, bromine content 65%) and brominated copolymer styrene and butadiene (EMERALD 3000, bromine content 64%) were obtained from Synthos, CZ.
Bromine content in flame retardant was determined by argentometric titration in Central Laboratories of University of Chemistry and Technology Prague.
Polymerization in test tubes
The polymerization mixture (approx. 20 g) was prepared under protective atmosphere of argon in a glass test tube (capacity 50 cm3, inner diameter 2 cm, length 15 cm). Fire retardant was dissolved or suspended in CL melt at 110 °C. Then, the activator (IPBCL or Bruggolen C20) was added and dissolved. Finally, the catalyst (CLMgBr, CLNa or DL) was dosed and dissolved. The test tube containing polymerization mixture was immersed in oil bath and kept 0.5 h at 150 °C (160 °C for DL).
Polymerization casting in mold
The polymerization mixture (approx. 100 g) was prepared under protective atmosphere of argon in Erlenmeyer glass flask. Fire retardant was dissolved or suspended in CL melt at 110 °C, and then the activator (IPBCL or Bruggolen C20) was dissolved. The catalyst (CLMgBr, CLNa or DL) was dosed, and after dissolution, the polymerization mixture was immediately transferred to the aluminum mold with wall thickness of 4 mm and internal cavity of 150(height) × 140 × 4 mm, sealed with a PTFE sealing tape. The mold was immersed in oil bath and kept at 150 °C (or 160 °C for DL) for 0.5 h and then left to cool at room temperature. Polymerization process was monitored by so-called temperature profile of polymerizing mixture via a thermocouple placed in the center of the mold. After demolding, test specimens have been prepared.
Characterization of polymer samples
A part of the crude polymer was disintegrated by a special rasp for the determination of water-extractable portion (WEP) by hot water extraction (3 × 20 min). The samples were dried at room temperature under reduced pressure (30 Pa) to constant mass.
Water-extractable portion (WEP) is calculated according to equation:
\({m}_{0}\)—mass of the sample before extraction and \(m\)—mass of the sample after extraction.
TGA measurements were carried out on a TGA Q500 (TA Instruments, USA) under a nitrogen atmosphere (gas flow 60 cm3 min−1) at a heating rate of 10 °C min−1 in a temperature range of 20–600 °C.
DSC measurements were carried out on DSC 250 (TA Instruments, USA). The mass of extracted sample was about 5–7 mg. Measurements were performed from room temperature to 240 °C under nitrogen purge and a heating/cooling rate of 10 °C min−1. Content of crystalline part is calculated according to the equation:
\({\Delta H}_\text{m}\)—enthalpy of fusion of PA6 phase determined by DSC, and \({\Delta H}_{100\%}\)—enthalpy of fusion of 100% crystalline PA6 is 190 J g−1, ref. [12].
Tensile tests were carried out on beam-shaped specimens (4 × 2 mm in cross section) cut from plate on INSTRON 3365 (Instron, USA). Clamp distance at Instron was 60 mm. Tensile tests were performed at a rate of 1 mm min−1 until the proportional elongation exceeded 1%. The displacement rate was 50 mm min−1.
Notch impact strength (ak) was measured using Charpy pendulum on specimens with dimensions of 50 × 20 × 4 mm and average notch depth 1 mm at 23 °C. The distance of supports was 40 mm, and pendulum velocity was 2.9 m s−1 on impact.
Determination flammability according to UL-94
Flammability tests were performed in a stainless steel chamber (Noselab ATS, Italy) with a volume of 1 m3 equipped with a Bunsen burner according to ASTM D 5025 with a power of 50 W. The standard test according to the ČSN EN 60695–11-10 ed.2 standard (UL 94) was performed on test specimens of dimensions 125 × 13 × 4 mm cut from plates prepared by polymerization casting.
Limiting oxygen index (LOI) was determined according to ISO 4589 in TIU Plast, CZ.
Results and discussion
From the list of flame retardants used for polyamides (especially polyamide 6) those that interact with components of the polymerization initiation system and/or growth centers—such as amino compounds, hydroxyl compounds and melamine—were excluded from this study.
Magnesium carbonate, magnesium hydroxide, aluminum oxide and antimony trioxide were tested as inorganic flame retardants. Commercially used compounds melamine phosphate, tris(tribromoneopentyl)phosphate (trade name FR-370), 1,1'-(isopropylidene)bis[3,5-dibromo-4-(2,3-dibromo-2-methylpropoxy)benzene (GC BDMP 66) and brominated styrene-butadiene copolymer (EMERALD 3000) were used as organic flame retardants, see Fig. 1.
Table 1 shows the bromine contents calculated from the chemical structure (excluding EMERALD 3000), declared by the manufacturer and determined by argentometric titration. There is a significant difference in the bromine content values for the “polymer” retardant EMERALD 3000.
Thermal stability of flame retardants
The retardants must be stable during the preparation of the polymerization handle (110 °C) and the polymerization process (bath temperature 160 °C) where the polymerization mixture can reach 200 °C due to the exothermic process, depending on the mass of the polymerization handle and the mold geometry. Therefore, all flame retardants were analyzed by TGA, see Figs. 2 and 3.
MgCO3 has a mass loss of 4.41% at 200 °C, and the decomposition product is CO2. Other mineral and all organic brominated flame retardants are stable up to 200 °C. Therefore, they should not interfere with the polymerization process by decomposition products unless they interact with the components of the polymerization system due to their chemical composition.
Test of initiation system of anionic polymerization of ε-caprolactam
Due to their chemical structure, flame retardants could act as polymerization inhibitors during the anionic polymerization of CL. Therefore, test polymerizations were carried out in test tubes with a capacity of about 20 g of polymerization mixture. Three commonly used initiation systems were used with component concentrations taken from the literature [13], see Table 2. In the following sections of this work, the initiation system will be referred to by the initiator abbreviation only.
A problem in anionic in situ intercalation polymerization was the sedimentation of inorganic flame retardants in the monomer melt – CL (m.p. = 70 °C). After complete melting of CL, sedimentation was observed mainly for MgCO3. A lower degree of sedimentation was observed for Mg(OH)2 and Al(OH)3. On the other hand, Sb2O3 did not sediment during the preparation of the polymerization mixture. Sedimentation was suppressed by vigorous stirring of the polymerization mixture at the beginning of the polymerization process until the viscosity of the polymerization mixture increased significantly with the onset of polymerization process. Therefore, stirring the polymerization mixture and increasing its viscosity during polymerization is an effective tool to suppress the sedimentation of the flame retardant. All organic flame retardants were soluble in the CL melt, and we assumed their uniform distribution in the polymerization mixture.
The effect of the flame retardant on the anionic polymerization process was evaluated by determining the content of water-extractable products (WEP) in the sample after polymerization (Table 2). These consist of unreacted CL, linear and cyclic oligomers. The inorganic and organic retarders are not water soluble. The criterion for the selection of the initiation system for the preparation of test samples for flammability tests was a WEP value of less than 5%. CLNa and DL polymerization initiators are more sensitive to the presence of impurities or inhibiting compounds than CLMgBr. Using these two initiators failed to prepare polymer containing MgCO3 due to CO2 release and for Mg(OH)2 due to water release. Carbon dioxide reacts with CLNa to form carboxylate [14]. When the concentration of carboxylate is lower than that of sodium salt in the polymerization mixture, the polymerization of CL is very low. Polymerization does not take place if CLNa is fully converted to carboxylate. Water is released from Mg(OH)2, and the presence of even a small amount of water in the reaction mixture adversely affects the course of the anionic polymerization, primarily by reducing the rate of polymerization, the degree of polymerization and the polymer yield [10].
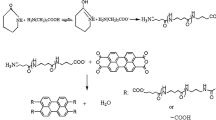
When CLNa and DL initiators were used, CL polymerization with presence of brominated organic flame retardants did not proceed, and no polymer was isolated (Table 2). The cause is due to nucleophilic substitution of brominated organic flame retardants (Fig. 4). By reacting the strong basic nucleophile CLNa (similarly DL) according to the first reaction, the equilibrium is shifted in favor of the product, i.e., the concentration of lactam anions necessary for repeated addition to N-acyllactam growth centers and formation of macromolecules (the second reaction) is greatly reduced in the system. This is not the case when using the CLMgBr initiator, which is a weak nucleophile, and the polymerization of CL in the presence of brominated derivatives proceeds to high conversion steps (low WEP value).
Polymerization casting in mold
Based on the results of test polymerizations, Table 2, PA6 plates containing flame retardant and reference PA6 were prepared, see Table 3. The concentrations of the components for CLNa/HDCL and CLMgBr/IPCL were left unchanged. For the DL/HDCL initiation system, a large delay between the start of polymerization and solidification of the polymerization mixture was observed for the tube polymerizations (see Table 2). Therefore, the polymerization temperature was increased from 150 °C to 160 °C, and the polymerization initiator concentration was increased to 0.33 mol% DL.
The dependence of the temperature of the polymerization mixture in the mold on the polymerization time was monitored during polymerization by a thermocouple. The time to reach the maximum polymerization temperature tp and an increase in the temperature ∆TP expressed as the difference between the maximum polymerization temperature and the oil bath temperature were evaluated. The data obtained, see Table 3 and Fig. 5, confirm that the polymerization rate depends on the initiator used and decreases in the CLMgBr, CLNa and DL series. The high polymerization rate with CLMgBr causes the highest increase in temperature of polymerizing mixture because the heat generated does not have time to be exchanged with the surrounding environment. For all dependencies, only one peak belonging to the temperature increase due to polymerization, and crystallization of the polymer was observed.
Temperature of the polymerization mixture (\({T}_\text{pm}\)) sensed by the thermocouple in the center of the mold on polymerization time; 5% Sb2O3, various initiators of CL polymerization, see Table 3
We were able to prepare plates with low WEP content ( < 3%) by polymerization casting, see Table 3. Only the material containing the polymer EMERALD 3000 had a WEP value = 8.6%, and the plate was inhomogeneous. The polymeric flame retardant is soluble in the melt of the monomer CL. However, during polymerization, the polymeric flame retardant and the resulting PA6 separate, and the two polymers are immiscible.
The filler sedimentation was determined by comparing the differences in the ash content (residue at 600 °C; TGA) of two samples taken from the top wr,T and bottom parts wr,B 10 cm apart of the molded plate (Table 4). Similarly, sedimentation of a filler was evaluated for PA 6 prepared by anionic polymerization of CL with dispersed montmorillonite [13].
At 600 °C, a decrease in the mass of Al(OH)3 and Mg(OH)2 is evident, see Fig. 2. Brominated organic flame retardants are unstable at this temperature, see Fig. 3.
The residue value of the samples at 600 °C is influenced by the filler content, its sedimentation and thermal stability. Very low sedimentation was observed for Sb2O3. The difference in uncombustible residue is at most 0.4%, and its total content is around 4.5%. Despite intensive mixing and timing of casting the polymerization mixture into the mold, sedimentation occurred for Al(OH)3 and Mg(OH)2 during (especially at the beginning of) polymerization. In general, the flame retardants used increased the extrapolated initial decomposition temperature, most significantly the aluminum and magnesium hydroxides. For the samples containing halogenated retardants, the shift in temperature at the maximum decomposition rate is toward a lower value compared to the other samples, including the reference sample.
The evaluation of the DSC records of the prepared materials is shown in Table 5. The melting temperatures of samples with brominated organic flame retardants are lower than those of samples with inorganic retardants. The presence of flame retardant increases the crystallization temperature compared to the reference sample PA6 (comparison for CLNa and DL initiators). On the second heating, both the melting temperature and the crystalline phase content were reduced.
Mechanical properties
The sample bars were prepared from the plates and subjected to tensile tests. The reference material was flame retardant-free polyamide 6 prepared by CL polymerization initiated by CLNa (see Table 3). In publication [15], the modulus (Et = 2.8 GPa) and toughness (ak = 3 kJ m−2) for PA 6 samples prepared under identical conditions are in good agreement with those for the reference sample.
From the results shown in Table 6, it is clear that the presence of flame retardants does not significantly affect the tensile modulus, which is around 3000 MPa. The tensile strength at break is also not affected and is around 82 MPa. Thus, no inorganic flame retardant shows a stiffening effect; this is due to its low content (5%). A decrease in elongation at break was observed. The presence of flame retardants also does not significantly affect the notch impact strength. The material containing EMERALD 3000 has significantly poorer mechanical properties for the reasons mentioned above, i.e., immiscibility of the polymeric flame retardant and the PA6 formed.
Flammability retardance tests
Vertical and horizontal flammability test was carried out according to the standard UL-94 on five specimens from each sample. The results of both tests are shown in Table 7.
The reference specimens, those containing Mg(OH)2 and Al(OH)3 and EMERALD 3000, were readily ignitable in the vertical flammability test, already burning strongly in the flame and continuing to burn after removal from the flame with minimal evidence of flame weakening for more than 60 s. This is due to the low 5% hydroxide concentration in the specimens, at which they are unable to extinguish the flame on their own. The reason for the combustion of the sample containing EMERALD 3000 is the presence of a larger amount of unreacted CL monomer, (the main component of WEP, see Table 3), which evaporates during the test and promotes combustion. The reference PA6 is characterized by a low melt viscosity, and the sample drips more rapidly during combustion and thus supports the combustion process.
Samples containing the brominated organic flame retardants FR-370 and GC BDMP 66 showed retardant effects and were classified as V-0. GC BDMP 66 appears to be the more suitable flame retardant due to the overall burn-up time, which was approximately twice as short as for FR-370. After the samples were removed from the flame, there was a flame flare-up, a subsequent drip that did not ignite the cotton wool, and an immediate extinction of the flame. Flame ignition is required to raise the temperature and produce HBr, which reacts with radicals in the gas phase, breaking the chain of thermo-oxidative radical reactions and extinguishing the flame.
Samples containing Sb2O3 exhibited inconsistent behavior. Samples prepared with CLNa and CLMgBr initiators burned for more than 60 s and were therefore not classified. The sample prepared with DL initiator was classified as V-2. During burning, dripping of flammable material occurred, which ignited the attached cotton wool. The difference in behavior is due to the different structure of the polymer chains. The solubility test of the samples in trifluoroethanol showed that the samples prepared with CLNa and CLMgBr initiators were soluble, and the samples prepared with DL were swollen in the solvent, i.e., partially crosslinked (swelling index 2850% and insoluble content 82%). The combination of the presence of Sb2O3 and the crosslinked PA6 structure was sufficient for a V-2 classification.
The horizontal flammability test complemented and confirmed the vertical test, see Table 7. The specimens classified by the vertical test did not continue to burn after the ignition source was removed. The other samples, including the reference, were classified as HB40 based on the observed linear burning rate. Only the sample containing EMERALD 3000 was classified as HB75. When comparing the two reference samples, the effect of the crosslinked PA6 structure was observed, which resulted in a lower linear burn rate for the DL-initiated sample compared to the CLNa-initiated sample. For the Al(OH)3/CLNa and Sb2O3/CLNa samples, there was a slight decrease in the linear burning rate compared to the reference PA6.
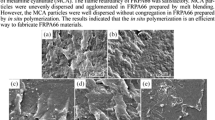
Examples of the charred residues from the vertical test are shown in Fig. 6. For the reference PA6, the ignited surface was clear and bubbled on ignition. In the samples containing inorganic hydroxides, bubbles also formed in the burning part, probably due to the decomposition of the hydroxide into water and its subsequent release. The formation of a black charred layer was observed in the samples containing Sb2O3. For FR-370 and GC BDMP 66, sparking in the flame occurred.
For the reference samples PA6 and for the samples classified by the vertical test (in categories V-0 and V-2), the oxygen number was determined, see Table 8. In practice, the oxygen number is not so widely used because it is determined at conditions that do not correspond to the actual burning conditions of the material. For this reason, the UL94 classification is preferred. The LOI value for PA6 without flame retardant was 21%, i.e., the same as the value reported in the literature [16]. For all samples containing flame retardant, the LOI values were around 24%, and its increase indicates the positive effect of flame retardants on reducing the flammability of PA6.
The current trend is to reduce brominated compounds in manufactured materials, so a concentration of 5 mass% could be problematic in this respect. For this reason, the samples classified by the vertical flammability test (FR-370/CLMgBr, GC BDMP 66/CLMgBr and Sb2O3/DL) were reduced in the flame retardant content in samples. Plates with flame retardant contents of 3 and 1 mass% were prepared by polymerization casting. These prepared samples were only subjected to a flammability test as no effect on thermal or mechanical properties was expected, see above.
Reducing the flame retardant content increases the burn-up times for the vertical test, see Table 9. All samples containing 3 mass% flame retardant were classified as V-2 due to the dripping melt that ignited the pulp. For the Sb2O3/DL and FR-370/CLMgBr samples, the attached pulp ignited each time. For GC sample BDMP 66/CLMgBr, the pulp ignited only a few times, but this also classifies this sample as V-2. During combustion, sparking in the flame was observed in all samples, in which reactions of the products formed with radicals in the gas phase took place. None of the samples containing 1 mass% of flame retardant were classified due to a first burn-up time longer than 60 s.
Conclusions
In summary, we succeeded in the preparation of polyamide 6 with reduced flammability by anionic polymerization of CL in the presence of flame retardants.
Based on test polymerizations, combinations of flame retardant and CL anionic polymerization initiation system were selected suitable for the preparation of PA6 in high yield by a method of polymerization casting. PA6 containing 5 mass% flame retardant was prepared by anionic polymerization of CL in the presence of organic brominated compounds under CLMgBr polymerization initiation, aluminum hydroxide and antimony trioxide under the initiation of polymerization with CLNa, DL and CLMgBr.
For PA6 containing 5 mass% flame retardant, there is no change in thermal stability, melting point and crystalline phase content. The presence of inorganic retardants accelerates the crystallization of PA6 from the melt. The values of tensile modulus, tensile strength at break and notch impact strength of the samples containing flame retardant are practically the same as those of the unfilled samples.
Materials containing 5% organic brominated flame retardants FR-370 and GC BDMP 66, both prepared under initiation of CL polymerization with CLMgBr, were classified as V-0 by the UL94 vertical burning test. The material containing 5 mass% Sb2O3 prepared by DL polymerization initiation was classified as V-2. The crosslinked structure of PA6 contributes to the retardation effect. The results of the vertical test were confirmed by the horizontal test. The LOI values of these three materials was around 24%, an increase over the reference PA6 (≈ 21%). Samples with reduced flame retardant content, i.e., 3% FR-370, GC BDMP 66 or Sb2O3 were categorized as V-2 by the vertical burn test.
Therefore, the polymer casting method of polyamide 6 will be further studied with the intention of balancing the type and concentration of flame retardant.
References
Puffr R, Kubánek V. Lactam-based Polyamides Polymerization, Structure, and Properties. 1st ed. Boca Raton: CRC Press, Boca Raton; 1991.
Gao J, Wu Y, Li J, Peng X, Yin D, Jin H, Wang S, Wang J, Wang X, Jin M, Yao Z. A review of the recent developments in flame-retardant nylon composites. Compos C Open Access. 2022. https://doi.org/10.1016/j.jcomc.2022.100297.
Kundu CK, Li Z, Song L, Hu Y. An overview of fire retardant treatments for synthetic textiles: from traditional approaches to recent applications. Eur Polym J. 2020. https://doi.org/10.1016/j.eurpolymj.2020.109911.
Coquelle M, Duquesne S, Casetta M, Sun J, Zhang S, Bourbigot S. Investigation of the decomposition pathway of polyamide 6/ammonium sulfamate fibers. Polym Degrad Stab. 2014. https://doi.org/10.1016/j.polymdegradstab.2014.02.007.
Gijsman P, Steenbakkers R, Fürst C, Kersjes J. Differences in the flame retardant mechanism of melamine cyanurate in polyamide 6 and polyamide 66. Polym Degrad Stab. 2002. https://doi.org/10.1016/S0141-3910(02)00136-2.
Levchik SV, Balabanovich AI, Levchik GF, Costa L. Effect of melamine and its salts on combustion and thermal decomposition of polyamide 6. Fire Mater. 1997. https://doi.org/10.1002/(SICI)1099-1018(199703)21:2%3C75::AID-FAM597%3E3.0.CO;2-P.
Levchik SV, Levchik GF, Camino G, Costa L, Lesnikovich AI. Mechanism of action of phosphorus-based flame retardants in nylon 6. III Ammonium Polyphosphate/Manganese Dioxide Fire Mater. 1996. https://doi.org/10.1002/(SICI)1099-1018(199607)20:4%3c183::AID-FAM574%3e3.0.CO;2-W.
Čolović M, Vasiljević J, Štirn Ž, Čelan Korošin N, Šobak M, Simončič B, Demšar A, Malucelli G, Jerman I. New sustainable flame retardant DOPO-NH-functionalized polyamide 6 and filament yarn. Chem Eng J. 2021. https://doi.org/10.1016/j.cej.2021.130760.
Wu ZY, Xu W, Xia JK, Liu YC, Wu QX, Xu WJ. Flame retardant polyamide 6 by in situ polymerization of ɛ-caprolactam in the presence of melamine derivatives. Chin Chem Lett. 2008. https://doi.org/10.1016/j.cclet.2007.12.012.
Bernat P, Hladká O, Fišmanová M, Roda J, Brožek J. Polymerization of lactams 98: Influence of water on the non-activated polymerization of ε-caprolactam. Eur Polym J. 2008. https://doi.org/10.1016/j.eurpolymj.2007.10.026.
Minář J, Brožek J, Michalcová A, Hadravová R, Slepička P. Functionalization of graphene oxide with poly(ε-caprolactone) for enhanced interfacial adhesion in polyamide 6 nanocomposites. Compos B Eng. 2019. https://doi.org/10.1016/j.compositesb.2019.107019.
Inoue M. Studies on crystallization of high polymers by differential thermal analysis. J Polym Sci A Gen Pap. 1963. https://doi.org/10.1002/pol.1963.100010813.
Kadlecová Z, Puffr R, Baldrian J, Schmidt P, Roda J, Brožek J. Homoionic inorganic montmorillonites as fillers for polyamide 6 nanocomposites. Eur Polym J. 2008. https://doi.org/10.1016/j.eurpolymj.2008.06.036.
Chrzczonowicz S, Wsłodarczyk M. Polymerization of ε-caprolactam in solvent. II. The infrared study of the mechanism of polymerization. Makromol Chem. 1961;48:135–43.
Bernášková A, Chromcová D, Brožek J, Roda J. Polymerization of lactams, 95 Preparation of polyesteramides by the anionic polymerization of ε-caprolactam in the presence of poly(ε-caprolactone). Polym. 2004. https://doi.org/10.1016/j.polymer.2004.01.034.
Lewin M, Brozek J, Martens MM. The system polyamide/sulfamate/dipentaerythritol: flame retardancy and chemical reactions. Polym Adv Technol. 2002. https://doi.org/10.1002/pat.243.
Acknowledgements
The authors thank the University of Chemistry and Technology, Prague, for financial support.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Contributions
JD involved in investigation, data curation, and writing—original draft. VB involved in methodology and investigation. JB involved in conceptualization, methodology, and writing—review and editing.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doležal, J., Benešová, V. & Brožek, J. Preparation of polyamide 6 with reduced flammability by polymerization casting. J Therm Anal Calorim 149, 981–991 (2024). https://doi.org/10.1007/s10973-023-12774-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12774-1