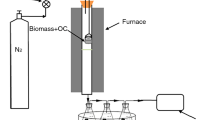
Abstract
Phosphogypsum (PG) can be reduced by thermal treatment and produces SO2 and lime slag. Unfortunately, the SO2 yield is low and the PG decomposition temperature is high. This problem can be resolved by adding reducing agents and additives. However, the current studies mainly investigate the effect of Fe3+ on the PG reduction in a fixed bed, while there are few studies on the preparation of SO2 from Fe2+-assisted coke reduction of PG in a fluidized bed and lack of analysis of the sulfur form in the gas products. Consequently, using coke as the reducing agent and Fe2+ as the additive, together with thermodynamic simulation and kinetic calculation, the impacts of Fe/Ca molar ratio, C/Ca molar ratio and reaction temperature on SO2 yield by PG decomposition in a fluidized bed are explored. It is found that the addition of Fe2+ could reduce reaction temperature and activation energy of the PG-C system. The inclusion of Fe2+ boosts the SO2 yield and PG decomposition rate in comparison to PG-C system. The SO2 yield and PG decomposition rate under these circumstances are 95.41% and 99.07%, respectively, with C/Ca of 0.5 and Fe/Ca of 1 at 1100 °C. The S in the gas products is in the form of SO2, COS and S2. Kinetic calculations reveal that the PG-C system and the PG-C-Fe2+ system are consistent with the nucleation and growth models with g(α) = -ln(1-α). The preparation of SO2 from PG reduction by Fe2+ synergistic coke is mainly achieved through the valence transition of Fe2+.
Graphical abstract











Similar content being viewed by others
References
Wu F, Ren Y, Qu G, Liu S, Chen B, Liu X. Utilization path of bulk industrial solid waste: A review on the multi-directional resource utilization path of phosphogypsum. J Environ Manage. 2022. https://doi.org/10.1016/j.jenvman.2022.114957.
Lv X, Xiang L. The generation process, impurity removal and high-value utilization of phosphogypsum material. Nanomaterials. 2022;12(17):3021. https://doi.org/10.3390/nano12173021.
Wei ZQ, Deng ZB. Research hotspots and trends of comprehensive utilization of phosphogypsum: Bibliometric analysis. J Environ Radioact. 2022. https://doi.org/10.1016/j.jenvrad.2021.106778.
Zheng S, Ning P, Ma L, Cheng F, Shi J. Phosphogypsum as a raw material for the production of SO2 and lime in circulating fluidized beds. Combust Sci Technol. 2014;186(3):377–86. https://doi.org/10.1080/00102202.2013.864286.
Ma D, Wang Q. Experimental study of CaS preparation from lignite-reduced phosphogypsum in a fluidized bed. J Chem Technol Biotechnol. 2022;98(3):756–72. https://doi.org/10.1002/jctb.7285.
Bi Y, Xu L, Yang M, Chen Q. Study on the effect of the activity of anthracite on the decomposition of phosphogypsum. Ind Eng Chem Res. 2022;61(19):6311–21. https://doi.org/10.1021/acs.iecr.2c00081.
Zheng SC, Ning P, Ma LP, Niu XK, Zhang W, Chen YH. Reductive decomposition of phosphogypsum with high-sulfur-concentration coal to SO2 in an inert atmosphere. Chem Eng Res Des. 2011;89(12A):2736–41. https://doi.org/10.1016/j.cherd.2011.03.016.
Suslikov AV, Zhirnov BS, Murtazin FR. A study of the kinetics of the reaction of petroleum coke with phosphogypsum to give calcium sulfide. Chem Technol Fuels Oils. 2021;57(3):461–6. https://doi.org/10.1007/s10553-021-01266-3.
Liu Q, Ao X, Chen Q, Xie Y, Cao Y. Reaction characteristics and kinetics of phosphogypsum decomposition via synergistic reduction effect of composite reducing agent. J Mater Cycles Waste Manage. 2022;24(2):595–605. https://doi.org/10.1007/s10163-021-01344-y.
Zhu K, Xie G, Chen Z, Wang Q. Reaction characteristics of phosphogypsum under carbon monoxide atmosphere. J Chin Ceram Soc. 2013;41(11):1540–5.
Yang X, Zhang Z, Wang X, Yang L, Zhong B, Liu J. Thermodynamic study of phosphogypsum decomposition by sulfur. J Chem Thermodyn. 2013;57:39–45. https://doi.org/10.1016/j.jct.2012.08.006.
Song W, Zhou J, Wang B, Li S, Cheng R. Production of SO2 gas: New and efficient utilization of flue gas desulfurization gypsum and pyrite resources. Ind Eng Chem Res. 2019;58(44):20450–60. https://doi.org/10.1021/acs.iecr.9b04403.
Lian Y, Ma L, Liu H, Tang J, Zhu B, Ma G, et al. Experimental study on preparation of calcium sulfide via phosphogypsum and hydrogen sulfide reaction. Chem Eng China. 2016;44(8):48–52.
Zheng D, Lu H, Sun X, Liu X, Han W, Wang L. Reaction mechanism of reductive decomposition of FGD gypsum with anthracite. Thermochim Acta. 2013;559:23–31. https://doi.org/10.1016/j.tca.2013.02.026.
Yang J, Zhu B, Ma L, Liu H. Investigation of Al2O3 and Fe2O3 transmission and transformation during the decomposition of phosphogypsum. Chin J Chem Eng. 2019;27(5):1125–31. https://doi.org/10.1016/j.cjche.2018.09.023.
Sun L, Zhao Z, Yang X, Sun Y, Li Q, Luo C. Thermochemical decomposition of phosphogypsum with Fe-P slag via a solid-state reaction. Chin J Chem Eng. 2022;47:113–9. https://doi.org/10.1016/j.cjche.2021.06.025.
Yan Z, Wang Z, Liu H, Tu Y, Yang W, Zeng H. Decomposition and solid reactions of calcium sulfate doped with SiO2, Fe2O3 and Al2O3. J Anal Appl Pyrolysis. 2015;113:491–8. https://doi.org/10.1016/j.jaap.2015.03.019.
Shi T, Wan T, Zhang Z, Yang X, Yang L, Zhong B. Effect of SiO2 on the melting characteristics of reaction between phosphogypsum and calcium sulfide. J Therm Anal Calorim. 2016;123(2):1601–9. https://doi.org/10.1007/s10973-015-5032-z.
Ennaciri Y, Bettach M, El Alaoui-Belghiti H. Phosphogypsum conversion into calcium fluoride and sodium sulfate. Annal De Chimie-Sci Des Materiaux. 2020;44(6):407–12. https://doi.org/10.18280/acsm.440606.
Ma LP, Du YL, Niu XK, Zheng SC, Zhang W. Thermal and kinetic analysis of the process of thermochemical decomposition of phosphogypsum with CO and additives. Ind Eng Chem Res. 2012;51(19):6680–5. https://doi.org/10.1021/ie2029859.
Yan B, Ma LP, Xie LG, Ma J, Zi ZC, Yan XD. Reaction mechanism for iron catalyst in the process of phosphogypsum decomposition. Ind Eng Chem Res. 2013;52(49):17383–9. https://doi.org/10.1021/ie402321w.
Zheng DL, Ma LP, Wang RM, Yang J, Dai QX. Decomposing properties of phosphogypsum with iron addition under two-step cycle multi-atmosphere control in fluidised bed. Waste Manage Res. 2018;36(2):183–93. https://doi.org/10.1177/0734242x17748362.
Xie LG, Ma LP, Dai QX, Mao Y, Zhang H, Ma J. Effects of additives on the reductive decomposition of phosphogypsum in the CO atmosphere. 2nd International conference on energy and environmental protection (ICEEP 2013); 2013 Apr 19–21; Guilin, PEOPLES R CHINA2013.
Di Z, Yang F, Cao Y, Zhang K, Guo Y, Gao S. The transformation pathways on the catalytic and stability-promoted CaSO4 reduction in CLC process using Fe2O3 supported. Fuel. 2019;253:327–38. https://doi.org/10.1016/j.fuel.2019.04.141.
Xia X, Zhang L, Li Z, Yuan X, Ma C, Song Z. Recovery of CaO from CaSO4 via CO reduction decomposition under different atmospheres. J Environ Manage. 2022. https://doi.org/10.1016/j.jenvman.2021.113855.
Sun XM, Huang WJ, Ji LP, Xu HM, Qu Z, Yan NQ. Establishing a self-supporting system of H2S production from SO2 with induced catalytic reduction process for mercury capture with super-large enrichment. Chem Eng J. 2023. https://doi.org/10.1016/j.cej.2023.141493.
Feng T, Zhou P, Zhao XQ, Li LZ, Xia X, Zhang SZ. Sulfur evolution reaction during reduction of SO2 with CO over carbon materials. Energy Fuels. 2019;33(8):7491–9. https://doi.org/10.1021/acs.energyfuels.9b00748.
Lu D, Chen Q, Li C, Gong S. Effect of potassium feldspar on the decomposition rate of phosphogypsum. J Chem Technol Biotechnol. 2021;96(2):374–83. https://doi.org/10.1002/jctb.6549.
Antar K, Jemal M. A thermogravimetric study into the effects of additives and water vapor on the reduction of gypsum and Tunisian phosphogypsum with graphite or coke in a nitrogen atmosphere. J Therm Anal Calorim. 2018;132(1):113–25. https://doi.org/10.1007/s10973-017-6871-6.
Funding
Thanks for the financial support from the school-enterprise cooperation project (No. 2019-KYY-508101–0078).
Author information
Authors and Affiliations
Contributions
Dong Ma contributed to experiment, formal analysis, data curation, investigation, and writing—original draft. Qinhui Wang contributed to review & editing, and supervision.
Corresponding author
Ethics declarations
Competing interests
This paper's authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10973_2023_12701_MOESM1_ESM.doc
XRD patterns of PG and FeCO3 (Fig. S1); ΔG versus temperature for each reaction (Fig. S2). Mechanism functions commonly used in solid phase reactions (Table S1) (DOC 359 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, D., Wang, Q. Preparation of sulfur dioxide by divalent iron synergistic coke reduction of phosphogypsum in a fluidized bed. J Therm Anal Calorim 148, 13959–13972 (2023). https://doi.org/10.1007/s10973-023-12701-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12701-4