Abstract
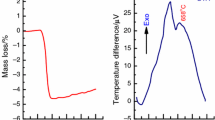
The mechanism of formation of nanocrystalline gadolinium orthoferrite (GdFeO3) during the heat treatment of gadolinium and iron(III) hydroxides synthesized by ultrasound-assisted co-precipitation was studied. The obtained samples were investigated by energy-dispersive X-ray spectroscopy, simultaneous thermal analysis via coupled differential scanning calorimetry and thermogravimetric analysis (DSC–TGA), powder X-ray diffraction (PXRD) and Fourier-transform infrared (FTIR) spectroscopy. The DSC–TGA results confirm that the formation of GdFeO3 occurs after the complete decomposition of gadolinium oxycarbonate derivatives. The PXRD results indicate that GdFeO3 is formed as a result of the reaction of amorphous iron(III) oxide (am-Fe2O3) at temperatures of 675–700 °C with amorphous gadolinium oxide (am-Gd2O3) (primary carbonate-independent pathway) and at temperatures of 725–775 °C with cubic gadolinium oxide (c-Gd2O3) transformed from the hexagonal gadolinium oxide (h-Gd2O3) which results from the decomposition of gadolinium oxycarbonate derivatives at temperatures of 675–725 °C (secondary carbonate-dependent pathway). The FTIR results are consistent with the assumption that gadolinium oxycarbonate derivatives decompose with the formation of h-Gd2O3 in the last-mentioned temperature range. The enthalpy of the reaction of formation of nanocrystalline GdFeO3 defined from the DSC–TGA data is equal to − 16.89 ± 0.36 kJ mol−1. The activation energy for the formation of nanocrystalline GdFeO3 obtained from the DSC data is equal to 1193.62 ± 112.05 kJ mol−1, 1202.27 ± 112.06 kJ mol−1 and 1151.08 ± 106.53 kJ mol−1 according to the Kissinger, Augis–Bennett/Boswell and Flynn–Wall–Ozawa methods, respectively. Also, based on the DSC data, the true onset temperature of the formation of nanocrystalline GdFeO3 was found to be ~ 756 °C.











Similar content being viewed by others
References
Bamzai KK, Bhat M. Electrical and magnetic properties of some rare earth orthoferrites (RFeO3 where R = Y, Ho, Er) systems. Integr Ferroelectr. 2014;158:108–22.
Zhou Z, Guo L, Yang H, Liu Q, Ye F. Hydrothermal synthesis and magnetic properties of multiferroic rare-earth orthoferrites. J Alloys Compd. 2014;583:21–31.
Ramu N, Muralidharan R, Meera K, Jeong YH. Tailoring the magnetic and magnetoelectric properties of rare earth orthoferrites for room temperature applications. RSC Adv. 2016;6:72295–9.
Nakhaei M, Sanavi KD. Study on structural, magnetic and electrical properties of ReFeO3 (Re= La, Pr, Nd, Sm & Gd) orthoferrites. Physica B Condens Matter. 2021;612: 412899.
Warshi MK, Mishra V, Sagdeo A, Mishra V, Kumar R, Sagdeo PR. Structural, optical and electronic properties of RFeO3. Ceram Int. 2018;44:8344–9.
Wang Z-Q, Lan Y-S, Zeng Z-Y, Chen X-R, Chen Q-F. Magnetic structures and optical properties of rare-earth orthoferrites RFeO3 (R = Ho, Er, Tm and Lu). Solid State Commun. 2019;288:10–7.
Sultan K, Samad R, Islam SAU, Habib MZ, Ikram M. Effect of rare earth ions (R = Pr, Eu and Ho) on the structural and electrical properties of orthoferrites. J Electron Mater. 2019;48:6003–7.
Yafarova LV, Chislova IV, Zvereva IA, Kryuchkova TA, Kost VV, Sheshko TF. Sol–gel synthesis and investigation of catalysts on the basis of perovskite-type oxides GdMO3 (M = Fe, Co). J Solgel Sci Technol. 2019;92:264–72. https://doi.org/10.1007/s10971-019-05013-3.
Niu X, Li H, Liu G. Preparation, characterization and photocatalytic properties of REFeO3 (RE=Sm, Eu, Gd). J Mol Catal A Chem. 2005;232:89–93.
Li L, Wang X, Lan Y, Gu W, Zhang S. Synthesis, photocatalytic and electrocatalytic activities of wormlike GdFeO3 nanoparticles by a glycol-assisted sol-gel process. Ind Eng Chem Res. 2013;52:9130–6. https://doi.org/10.1021/ie400940g.
Zhang Y, Zheng A, Yang X, He H, Fan Y, Yao C. Cubic GdFeO3 particle by a simple hydrothermal synthesis route and its photoluminescence and magnetic properties. CrystEngComm. 2012;14:8432.
Niu X, Du W, Du W. Preparation, characterization and gas-sensing properties of rare earth mixed oxides. Sens Actuators B Chem. 2004;99:399–404.
Söderlind F, Fortin MA, Petoral RM Jr, Klasson A, Veres T, Engström M, et al. Colloidal synthesis and characterization of ultrasmall perovskite GdFeO3 nanocrystals. Nanotechnology. 2008;19: 085608.
Pinho SLC, Amaral JS, Wattiaux A, Duttine M, Delville M-H, Geraldes CFGC. Synthesis and characterization of rare-earth orthoferrite LnFeO3 nanoparticles for bioimaging. Eur J Inorg Chem. 2018;2018:3570–8. https://doi.org/10.1002/ejic.201800468.
Athar T, Vishwakarma SK, Bardia A, Khan AA. Super paramagnetic iron oxide and gadolinium (FeGdO3) nanopowder synthesized by hydrolytic approach passes high level of biocompatibility and MRI-based dual contrast property for competent molecular imaging and therapeutic interventions. Biomed Phys Eng Express. 2016;2:025010. https://doi.org/10.1088/2057-1976/2/2/025010.
Deka S, Saxena V, Hasan A, Chandra P, Pandey LM. Synthesis, characterization and in vitro analysis of α-Fe2O3–GdFeO3 biphasic materials as therapeutic agent for magnetic hyperthermia applications. Mater Sci Eng C. 2018;92:932–41.
Abiev RSh, Almjasheva OV, Popkov VI, Proskurina OV. Microreactor synthesis of nanosized particles: the role of micromixing, aggregation, and separation processes in heterogeneous nucleation. Chem Eng Res Des. 2022;178:73–94.
Mariyappan V, Keerthi M, Chen S-M, Jeyapragasam T. Nanostructured perovskite type gadolinium orthoferrite decorated RGO nanocomposite for the detection of nitrofurantoin in human urine and river water samples. J Colloid Interface Sci. 2021;600:537–49.
Ateia EE, Hussein B, Singh C, Okasha N. Study of physical properties of Co substituted GdFeO3 orthoferrites and evaluation of their antibacterial activity. J Inorg Organomet Polym Mater. 2020;30:4320–8.
Santhosh BS, Yashas SR, Kumara Swamy N, Shivaraju HP. Application of non-hierarchical gadolinium ortho-ferrite nanostructure for LED-driven photocatalytic mineralization of doxycycline hydrochloride. J Mater Sci: Mater Electron. 2022;33:11676–86.
Tang P, Hu Y, Lin T, Jiang Z, Tang C. Preparation of nanocrystalline GdFeO3 by microwave method and its visible-light photocatalytic activity. Integr Ferroelectr. 2014;153:73–8. https://doi.org/10.1080/10584587.2014.902720.
Sivakumar M, Gedanken A, Bhattacharya D, Brukental I, Yeshurun Y, Zhong W, et al. Sonochemical synthesis of nanocrystalline rare earth orthoferrites using Fe(CO)5 precursor. Chem Mater. 2004;16:3623–32. https://doi.org/10.1021/cm049345x.
Mathur S, Shen H, Lecerf N, Kjekshus A, Fjellvåg H, Goya GF. Nanocrystalline orthoferrite GdFeO3 from a novel heterobimetallic precursor. Adv Mater. 2002;14:1405–9.
Lone IH, Khan H, Jain AK, Ahmed J, Ramanujachary KV, Ahmad T. Metal-organic precursor synthesis, structural characterization, and multiferroic properties of GdFeO3 nanoparticles. ACS Omega. 2022;7:33908–15.
Albadi Y, Martinson KD, Shvidchenko AV, Buryanenko IV, Semenov VG, Popkov VI. Synthesis of GdFeO3 nanoparticles via low-temperature reverse co-precipitation: the effect of strong agglomeration on the magnetic behavior. Nanosyst Phys Chem Math. 2020;11:252–9.
Albadi Y, Sirotkin AA, Semenov VG, Abiev RS, Popkov VI. Synthesis of superparamagnetic GdFeO3 nanoparticles using a free impinging-jets microreactor. Russ Chem Bull. 2020;69:1290–5. https://doi.org/10.1007/s11172-020-2900-x.
Popkov VI, Albadi Y. The effect of co-precipitation temperature on the crystallite size and aggregation/agglomeration of GdFeO3 nanoparticles. Nanosyst Phys Chem Math. 2021;12:224–31.
Albadi Y, Ivanova MS, Grunin LY, Martinson KD, Chebanenko MI, Izotova SG, et al. The influence of co-precipitation technique on the structure, morphology and dual-modal proton relaxivity of GdFeO3 nanoparticles. Inorganics (Basel). 2021;9:39.
Albadi Y, Abiev RS, Sirotkin AA, Martinson KD, Chebanenko MI, Nevedomskiy VN, et al. Physicochemical and hydrodynamic aspects of GdFeO3 production using a free impinging-jets method. Chem. Eng. Process.: Process Intensif. 2021;166: 108473.
Albadi Y, Ivanova MS, Grunin LY, Makarin RA, Komlev AS, Chebanenko MI, et al. Ultrasound-assisted co-precipitation synthesis of GdFeO3 nanoparticles: structure, magnetic and MRI contrast properties. Phys Chem Chem Phys. 2022;24:29014–23.
Prakash BJ, Rudramadevi BH, Buddhudu S. Analysis of ferroelectric, dielectric and magnetic properties of GdFeO3 nanoparticles. Ferroelectr Lett Sect. 2014;41:110–22. https://doi.org/10.1080/07315171.2014.956020.
Tugova EA, Karpov ON. Nanocrystalline perovskite-like oxides formation in Ln2O3–Fe2O3–H2O (Ln = La, Gd) systems. Nanosyst Phys Chem Math. 2014;5:854–60.
Popkov VI, Tugova EA, Bachina AK, Almyasheva OV. The formation of nanocrystalline orthoferrites of rare-earth elements XFeO3 (X = Y, La, Gd) via heat treatment of coprecipitated hydroxides. Russ J Gen Chem. 2017;87:2516–24. https://doi.org/10.1134/S1070363217110020.
Natarajan M, Secco EA. Anisotropic conductivity and phase transformation studies in potassium chromate crystals. Can J Chem. 1974;52:2436–8.
Glushko VP, editor. Thermal constants of substances. handbook in 10 Issues. Issue 8. Part 1. Tables of accepted values. Moscow: USSR Academy of Sciences. All-Union Institute of Scientific and Technical Information. Institute for High Temperatures; 1978.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Augis JA, Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal. 1978;13:283–92.
Boswell PG. On the calculation of activation energies using a modified Kissinger method. J Therm Anal. 1980;18:353–8.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci B. 1966;4:323–8.
Zhang X. Applications of kinetic methods in thermal analysis: a review. Eng Sci. 2021;14:1–13.
Zinkevich M. Thermodynamics of rare earth sesquioxides. Prog Mater Sci. 2007;52:597–647.
Zhang FX, Lang M, Wang JW, Becker U, Ewing RC. Structural phase transitions of cubic Gd2O3 at high pressures. Phys Rev B. 2008;78: 064114.
Ge W, Li Z, Lei Z, Chen T, Fu Z, Peng R, et al. Synthesis of hexagonal phase Gd2O2CO3: Yb3+, Er3+upconversion nanoparticles via SiO2 coating and Nd3+ doping. CrystEngComm. 2015;17:5702–9.
Gaspar RDL, Mazali IO, Sigoli FA. Particle size tailoring and luminescence of europium(III)-doped gadolinium oxide obtained by the modified homogeneous precipitation method: Dielectric constant and counter anion effects. Colloids Surf A Physicochem Eng Asp. 2010;367:155–60.
Sai Vandana C, Hemalatha Rudramadevi B. Effect of Cu2+ substitution on the structural, magnetic and electrical properties of gadolinium orthoferrite. Mater Res Express. 2018;5(4): 046101. https://doi.org/10.1088/2053-1591/aab7a8
Acknowledgements
The study was partially performed using the equipment of the Engineering Center of Saint Petersburg State Institute of Technology.
Author information
Authors and Affiliations
Contributions
Y. Albadi and V. I. Popkov contributed to conceptualization, methodology, validation and formal analysis; Y. Albadi and A. K. Bachina did investigation; A. K. Bachina and V. I. Popkov helped in resources; Y. Albadi did data curation, writing—original draft, visualization and writing—review & editing; V. I. Popkov contributed to writing—review & editing, supervision, project administration and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albadi, Y., Bachina, A.K. & Popkov, V.I. Physicochemical processes and thermochemical parameters of GdFeO3 formation from amorphous hydroxides: decisive role of carbonate impurities. J Therm Anal Calorim 148, 13281–13295 (2023). https://doi.org/10.1007/s10973-023-12647-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12647-7