Abstract
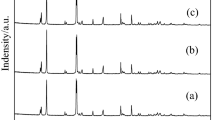
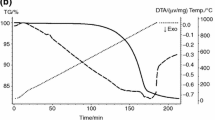
Jarosite is a specific type of hazardous waste determined by the origin of the primary raw materials of the hydrometallurgical process of zinc production. It represents a very demanding raw material for further treatment and recover of valuable metals due to their complex phase structures. The aim of this study was to collect relevant data for the designing the process of thermal decomposition of jarosite Pb–Ag sludge desired structure, which includes the transformation of Fe(II) and Fe(III) sulfates to insoluble hematite, retention of Cu, Zn and In in the form of soluble sulfates, with the possibility of their subsequent valorization by hydrometallurgical methods. Thermal decomposition of the industrial sample of jarosite Pb–Ag sludge is investigated under different temperatures (670–750 °C) and atmospheres (air and nitrogen) by thermodynamic, thermogravimetric and kinetic analysis. Physicochemical characterization of samples was done by ICP-MS, AAS, IMS, XRD, SEM techniques. The examined sample is ammonium jarosite type with the dominant phases NH4Fe3(SO4)2(OH)6 × H2O (44.00%) and ZnFe2O4 (15.25%). Experimental results, physicochemical characterization and thermodynamic analysis confirmed that the maximum content of hematite (Fe2O3) and metal sulfates (Cu, Pb and Zn), as required compounds for selective metal extraction, without the presence of unreacted ammonium jarosite, was obtained at 730 °C. A kinetic study obtained by thermogravimetric analysis showed that the thermal decomposition of jarosite Pb–Ag sludge takes place in two phases. The activation energy, calculated using the Kissinger–Akahira–Sunose iso-conversion method, was 235.4 kJ mol−1 and 208.8 kJ mol−1 for the decomposition of jarosite in air and nitrogen, respectively.










Similar content being viewed by others
References
Han H, Sun W, Hu Y, Jia B, Tang H. Anglesite and silver recovery from jarosite residues through roasting and sulfidization-flotation in zinc hydrometallurgy. J Hazard Mater. 2014. https://doi.org/10.1016/j.jhazmat.2014.05.091.
Pappu A, Saxena M, Asolekar SR. Jarosite characteristics and its utilization potentials. Sci Total Environ. 2006. https://doi.org/10.1016/j.scitotenv.2005.04.024.
Cruells M, Roca A. Jarosites: formation, structure reactivity and environmental. Metals. 2022. https://doi.org/10.3390/met12050802.
Zhu D, Yang C, Pan J, Guo Z, Li S. New pyrometallurgical route for separation and recovery of Fe, Zn, In, Ga and S from jarosite residues. J Clean Prod. 2018. https://doi.org/10.1016/j.jclepro.2018.09.152.
Wang Y, Yang H, Zhang W, Song R, Jiang B. Study on recovery of lead, zinc, iron from jarosite residues and simultaneous sulfur fixation by direct reduction. Physicochem ProblMiner Process. 2018;54(2):517–26.
Rämä M, Nurmi S, Jokilaakso A, Klemettinen L, Taskinen P, Salminen J. Thermal processing of jarosite leach residue for a safe disposable slag and valuable metals recovery. Metals. 2018. https://doi.org/10.3390/met8100744.
Tang L, Tang C, Xiao J, Zeng P, Tang M. A cleaner process for valuable metals recovery from hydrometallurgical zinc residue. J Clean Prod. 2018. https://doi.org/10.1016/j.jclepro.2018.08.096.
Grudinsky P, Pankratov D, Kovalev D, Grigoreva D, Dyubanov V. Comprehensive study on the mechanism of sulfating roasting of zinc plant residue with iron sulfates. Materials. 2021. https://doi.org/10.3390/ma14175020.
Ju S, Zhang Y, Zhang Y, Xue P, Wang Y. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy. J Hazard Mater. 2011. https://doi.org/10.1016/j.jhazmat.2011.05.049.
Wang Y, Yang H, Zhang G, Kang J, Wang C. Comprehensive recovery and recycle of jarosite residues from zinc hydrometallurgy. Chem Eng J Adv. 2020. https://doi.org/10.1016/j.ceja.2020.100023.
Frost RL, Wills RA, Weier ML, Musumeci AW, Martens W. Thermal decomposition of natural and synthetic plumbojarosite: importance in “archeochemistry.” Thermochim Acta. 2005. https://doi.org/10.1016/j.tca.2005.04.001.
Frost R, Wills RA, Kloprogge J, Martens W. Thermal decomposition of ammonium jarosite (NH4) Fe3(SO4)2(OH) 6. J Therm Anal Calorim. 2006. https://doi.org/10.1007/s10973-005-6953-8.
Flores MU, Reyes IA, Palacios EG, Patiño F, Juárez JC, Reyes M, Teja AM, Islas H, Gutiérrez EJ. Kinetic analysis of the thermal decomposition of a synthetic mercury jarosite. Minerals. 2019. https://doi.org/10.3390/min9040200.
Ma X, Tan H, Liu J, Wang J, He X. Preparation of ammonium jarosite and estimated activation energy of thermal decomposition in reducing atmosphere. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-018-7441-2.
Mustafa MK, Mandić V, Ćurković L, Šipušić J. Investigation of thermal decomposition of jarosite tailing waste. J Therm Anal Calorim. 2016. https://doi.org/10.1007/s10973-015-4881-9.
Ma X, Tan H, Dong F, Li B, Liu J, Chen Y, Wang L. Preparation of pyrrhotite from ammonium jarosite and estimation of activation energy in reducing atmosphere. Int J Chem React Eng. 2019. https://doi.org/10.1515/ijcre-2018-0149.
Kamberović Ž, Gajić N, Korać M, Jevtić S, Sokić M, Stojanović J. Technologically sustainable route for metals valorization from Jarosite-P–Ag sludge. Minerals. 2021. https://doi.org/10.3390/min11030255.
Roine A. HSC Chemistry® v. 9 [Software]. Outotec Research Oy Center: Pori, Finland. 2016.
Match! Software version 3.15 Build 270. https://www.crystalimpact.com/match/download.htm#download.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011. https://doi.org/10.1016/j.tca.2011.03.034.
Vyazovkin S, Burnham AK, Favergeon L, Koga N, Moukhina E, Pérez-Maqueda LA, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for analysis of multi-step Kinetics. Thermochim Acta. 2020. https://doi.org/10.1016/j.tca.2020.178597.
Steinlechner S, Antrekowitsch J. Thermodynamic considerations for a pyrometallurgical extraction of indium and silver from a jarosite residue. Metals. 2018. https://doi.org/10.3390/met8050335.
Hoeber L, Steinlechner S. A comprehensive review of processing strategies for iron precipitation residues from zinc hydrometallurgy. Clean Eng Technol. 2021. https://doi.org/10.1016/j.clet.2021.100214.
Linsong W, Peng Z, Yu F, Sujun L, Yue Y, Li W, Wei S. Recovery of metals from jarosite of hydrometallurgical nickel production by thermal treatment and leaching. Hydrometallurgy. 2020. https://doi.org/10.1016/j.hydromet.2020.105493.
Shi S, Zhou X, Chen W, Wang X, Nguyen T, Chen M. Thermal and kinetic behaviors of fallen leaves and waste tires using thermogravimetric analysis. BioResources. 2017. https://doi.org/10.15376/biores.12.3.4707-4721.
Heydari M, Rahman M, Gupta R. Kinetic study and thermal decomposition behavior of lignite coal. Int J Chem Eng. 2015. https://doi.org/10.1155/2015/481739.
Acknowledgements
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Contract No. 451-03-47/2023-01/200135 and 451-03-47/2023-01/200287)
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Željko Kamberović, Milisav Ranitović, Vaso Manojlović and Sanja Jevtić, The first draft of the manuscript was written by Marija Štulović and Nataša Gajić and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamberović, Ž., Ranitović, M., Manojlović, V. et al. Thermodynamic and kinetic analysis of jarosite Pb–Ag sludge thermal decomposition for hydrometallurgical utilization of valuable elements. J Therm Anal Calorim 148, 11799–11810 (2023). https://doi.org/10.1007/s10973-023-12508-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12508-3