Abstract
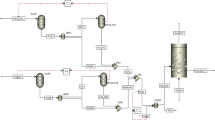
Using the coal spontaneous combustion experiment platform as the experimental testing method, based on Dafosi (defined as DFS) coal sample and Dongtan (defined as DT) coal sample, the characteristic temperature of coal spontaneous combustion was found out through a series of parameters such as heating rate, oxygen consumption rate, gas generation rate, single gas and composite gas index, and the corresponding relationship between characteristic temperature and each parameter was analyzed, the corresponding relationship between coal temperature and relevant parameters of gas generation is established, and the characteristic temperature of coal spontaneous combustion is determined as T1 = 35 °C, T2 = 55 °C, T3 = 75 °C, T4 = 100 °C, T5 = 145 °C, T6 = 190 °C, T7 = 230 °C, T8 = 310 °C.








Similar content being viewed by others
References
Bhoi S, Banerjee T, Mohanty K. Molecular dynamic simulation of spontaneous combustion and pyrolysis of brown coal using ReaxFF. Fuel. 2014;136:326–33.
Deng J, Xiao Y, Li Q, Junhui Lu, Wen Hu. Experimental studies of spontaneous combustion and anaerobic cooling of coal. Fuel. 2015;157:261–9.
Deng J, Zhao J, Zhang Y, Huang A, Liu X, Zhai X, Wang C. Thermal analysis of spontaneous combustion behavior of partially oxidized coal. Process Saf Environ Prot. 2016;104:218–24.
Lei C, Deng J, Cao K, Ma Li, Xiao Y, Ren L. A random forest approach for predicting coal spontaneous combustion. Fuel. 2018;223:63–73.
Liu W, Qin Y. Multi-physics coupling model of coal spontaneous combustion in longwall gob area based on moving coordinates. Fuel. 2017;188:553–66.
Tan B, Zhu H, Haiyan W, Hao Y, Jia G. Prediction model of coal spontaneous combustion critical point and the characteristics of adiabatic oxidation phase. J China Coal Soc. 2013;38(1):38–43.
Tripathi DD. New approaches for increasing the incubation period of spontaneous combustion of coal in an underground mine panel. Fire Technol. 2008;44(2):185–98.
Wang D, Xin H, Qi X, Dou G, Qi G, Ma L. Reaction pathway of coal oxidation at low temperatures: a model of cyclic chain reactions and kinetic characteristics. Combust Flame. 2016;163:447–60.
Wen Hu, Jun-hui Lu, Xiao Y, Deng J. Temperature dependence of thermal conductivity, diffusion and specific heat capacity for coal and rocks from coalfield. Thermochim Acta. 2015;619:41–7.
Wen H, Wang H, Liu W, Cheng X. Comparative study of experimental testing methods for characterization parameters of coal spontaneous combustion. Fuel. 2020;275:117880.
Wen H, Zhijin Y, Deng J, Zhai X. Spontaneous ignition characteristics of coal in a large-scale furnace: an experimental and numerical investigation. Appl Therm Eng. 2017;114:583–92.
Wen H, Zhijin Y, Fan S, Zhai X, Liu W. Prediction of spontaneous combustion potential of coal in the gob area using CO extreme concentration: a case study. Combust Sci Technol. 2017;189(10):1713–27.
Xiao Y, Lü H-F, Yi X, Deng J, Shu C-M. Treating bituminous coal with ionic liquids to inhibit coal spontaneous combustion. J Therm Anal Calorim. 2018;135(5):2711–21.
Yang Y, Li Z, Si L, Hou S, Li Z, Li J. Study on test method of heat release intensity and thermophysical parameters of loose coal. Fuel. 2018;229:34–43.
Zhang Y, Wang J, Xue S, Yue Wu, Li Z, Chang L. Evaluation of the susceptibility of coal to spontaneous combustion by a TG profile subtraction method. Korean J Chem Eng. 2016;33(3):862–72.
Zhu H, Wang H, Song Z, He C. The relationship between oxidation kinetics characteristic parameters of coal adiabatic progress and metamorphic degree. J China Coal Soc. 2014;39(3):498–503.
Zubíček V, Adamus A. Susceptibility of coal to spontaneous combustion verified by modified adiabatic method under conditions of Ostrava-Karvina Coalfield, Czech Republic. Fuel Process Technol. 2013;113:63–6.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 52174200), National Key R&D Projects (Programs 2016YFC0801802 and 2018YFC0808201) and Gansu Youth Science and Technology Fund (23JRRA833).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Fei, J., Luo, Z. et al. Experimental study on characteristic temperature of coal spontaneous combustion. J Therm Anal Calorim 148, 10011–10019 (2023). https://doi.org/10.1007/s10973-023-12365-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12365-0