Abstract
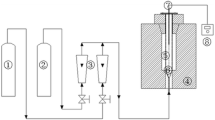
In this work, the chemical preparation of activated carbon (AC) using walnut shell (WS) and plastic wastes (primarily consisting of polyethylene, polypropylene, and polystyrene) as feedstocks and chemical activators (KOH and NaOH) was investigated in a fixed-bed reactor. Thermogravimetric analysis (TG) (TGA) was used to carbonize WS together with plastic waste. Furthermore, the Brunauer–Emmett–Teller (BET) method, FTIR, dye adsorption, iodine number, and SEM experiments were applied to characterize the obtained ACs. The results indicated that the polyolefinic waste acted as insulation and controlled the energy reaching WS particles to a certain extent. In addition, the reactive pyrolysis products of plastics such as styrene and light olefins reacted with the unstable structure of WS and intensified the secondary reactions during the carbonization process. Secondary reactions led to the creation of new structures that were clearly visible in the FTIR spectra. Also, the results indicated that the participation of plastics in secondary reactions has led to an increase in AC production. Furthermore, during the activation process, plastics have led to a significant increase in the surface area and volume of pores. The curve of WS degradation together with plastic waste showed that plastics have slowed down the WS degradation, as indicated by the slope of the degradation graph has clearly decreased.
Graphical abstract






Similar content being viewed by others
References
Bernal V, Giraldo L, Moreno-Piraján JC. Thermodynamic study of triclosan adsorption from aqueous solutions on activated carbon: modelling of experimental adsorption isotherm and calorimetry data. J Therm Anal Calorim. 2020;139:913–21.
Tabak A, Sevimli K, Kaya M, Çağlar B. Preparation and characterization of a novel activated carbon component via chemical activation of tea woody stem. J Therm Anal Calorim. 2019;138:3885–95.
Menya E, Olupot P, Storz H, Lubwama M, Kiros Y, John M. Effect of alkaline pretreatment on the thermal behavior and chemical properties of rice husk varieties in relation to activated carbon production. J Therm Anal Calorim. 2020;139:1681–91.
Zhou X-M, Liu Y-F. Study on the preparation of high adsorption activated carbon material and its application as phase change energy storage carrier material. J Therm Anal Calorim. 2022. https://doi.org/10.1007/s10973-021-11122-5.
Li Y, Lin Y, Xu Z, Wang B, Zhu T. Oxidation mechanisms of H2S by oxygen and oxygen-containing functional groups on activated carbon. Fuel Process Technol. 2019;189:110–9.
Al-Musawi TJ, McKay G, Kadhim A, Joybari MM, Balarak D. Activated carbon prepared from hazelnut shell waste and magnetized by Fe3O4 nanoparticles for highly efficient adsorption of fluoride. Biomass Convers Biorefinery. 2022. https://doi.org/10.1007/s13399-022-02593-z.
Abbaci F, Nait-Merzoug A, Guellati O, Harat A, El Haskouri J, Delhalle J, et al. Bio/KOH ratio effect on activated biochar and their dye based wastewater depollution. J Anal Appl Pyrol. 2022;162:105452.
Stano G, Di Nisio A, Lanzolla AM, Ragolia M, Percoco G. Fused filament fabrication of commercial conductive filaments: experimental study on the process parameters aimed at the minimization, repeatability and thermal characterization of electrical resistance. Int J Adv Manuf Technol. 2020;111(9):2971–86.
Zhong L, Zhang Y, Wang T, Ji Y, Norris P, Pan W-P. Optimized methods for preparing activated carbon from rock asphalt using orthogonal experimental design. J Therm Anal Calorim. 2019;136:1989–99.
Ismail IS, Singh G, Smith P, Kim S, Yang J-H, Joseph S, et al. Oxygen functionalized porous activated biocarbons with high surface area derived from grape marc for enhanced capture of CO2 at elevated-pressure. Carbon. 2020;160:113–24. https://doi.org/10.1016/j.carbon.2020.01.008.
Liu X, Zuo S, Cui N, Wang S. Investigation of ammonia/steam activation for the scalable production of high-surface area nitrogen-containing activated carbons. Carbon. 2022;191:581–92. https://doi.org/10.1016/j.carbon.2022.02.014.
Heidarinejad Z, Dehghani MH, Heidari M, Javedan G, Ali I, Sillanpää M. Methods for preparation and activation of activated carbon—a review. Environ Chem Lett. 2020;18(2):393–415.
Bazan A, Nowicki P, Półrolniczak P, Pietrzak R. Thermal analysis of activated carbon obtained from residue after supercritical extraction of hops. J Therm Anal Calorim. 2016;125:1199–204.
Gao Y, Yue Q, Gao B, Li A. Insight into activated carbon from different kinds of chemical activating agents—a review. Sci Total Environ. 2020;746:141094.
Shawabkeh RA, Aslam Z, Hussien IA. Thermochemical treatment of fly ash for synthesis of mesoporous activated carbon. J Therm Anal Calorim. 2015;122:1191–201.
Enterría M, Martín-Jimeno FJ, Suárez-García F, Paredes JI, Pereira MFR, Martins JI, et al. Effect of nanostructure on the supercapacitor performance of activated carbon xerogels obtained from hydrothermally carbonized glucose-graphene oxide hybrids. Carbon. 2016;105:474–83. https://doi.org/10.1016/j.carbon.2016.04.071.
Oishi S, Amano Y, Aikawa M, Machida M. Adsorption of Pb(II) ion on mesoporous activated carbon prepared by ZnCl2 activation. Carbon. 2012;50(3):1445. https://doi.org/10.1016/j.carbon.2011.11.023.
Giraldo L, Moreno-Piraján JC. CO2 adsorption on activated carbon prepared from mangosteen peel: study by adsorption calorimetry. J Therm Anal Calorim. 2018;133:337–54.
de la Torre-Miranda N, Reilly L, Eloy P, Poleunis C, Hermans S. Thiol functionalized activated carbon for gold thiosulfate recovery, an analysis of the interactions between gold and sulfur functions. Carbon. 2023;204:254–67. https://doi.org/10.1016/j.carbon.2022.12.061.
Serafin J, Dziejarski B, Cruz Junior OF, Sreńscek-Nazzal J. Design of highly microporous activated carbons based on walnut shell biomass for H2 and CO2 storage. Carbon. 2023;201:633–47. https://doi.org/10.1016/j.carbon.2022.09.013.
Hassan MF, Sabri MA, Fazal H, Hafeez A, Shezad N, Hussain M. Recent trends in activated carbon fibers production from various precursors and applications—a comparative review. J Anal Appl Pyrol. 2020;145:104715.
Abdul Khalil H, Firoozian P, Jawaid M, Akil H, Hassan A. Preparation of activated carbon filled epoxy nanocomposites: morphological and thermal properties. J Therm Anal Calorim. 2013;113:623–31.
Abbas-Abadi MS. The effect of process and structural parameters on the stability, thermo-mechanical and thermal degradation of polymers with hydrocarbon skeleton containing PE, PP, PS, PVC, NR, PBR and SBR. J Therm Anal Calorim. 2021;143(4):2867–82. https://doi.org/10.1007/s10973-020-09344-0.
Salas-Enríquez BG, Torres-Huerta AM, Conde-Barajas E, Domínguez-Crespo MA, Díaz-García L, Negrete-Rodríguez MDLLX. Activated carbon production from the Guadua amplexifolia using a combination of physical and chemical activation. J Therm Anal Calorim. 2016;124:1383–98.
Pamphile N, Xuejiao L, Guangwei Y, Yin W. Synthesis of a novel core-shell-structure activated carbon material and its application in sulfamethoxazole adsorption. J Hazard Mater. 2019;368:602–12.
Amorós-Pérez A, Cano-Casanova L, Ouzzine M, Rufete-Beneite M, Romero-Anaya AJ, Lillo-Ródenas MÁ, et al. Spherical activated carbons with high mechanical strength directly prepared from selected spherical seeds. Materials. 2018;11(5):770.
Arantes HT, Machado MA, Santoro MC, Freitas JC, Ronconi CM, Ligiero CB, et al. Effect of activated biochar as a low-cost catalyst on the quality of catalytic intermediate co-pyrolysis oil from waste polystyrene and green coconut pericarp. Fuel Process Technol. 2023;240:107539.
Tazibet S, Velasco L, Lodewyckx P, Abou M’Hamed D, Boucheffa Y. Systematic study of the role played by ZnCl2 during the carbonization of a chemically activated carbon by TG–MS and DSC. J Therm Anal Calorim. 2018;134:1395–404.
Heidari A, Khaki E, Younesi H, Lu HR. Evaluation of fast and slow pyrolysis methods for bio-oil and activated carbon production from eucalyptus wastes using a life cycle assessment approach. J Clean Prod. 2019;241:118394.
Abbas-Abadi MS, Van Geem KM, Alvarez J, Lopez G. The pyrolysis study of polybutadiene rubber under different structural and process parameters: comparison with polyvinyl chloride degradation. J Therm Anal Calorim. 2022;147(2):1237–49. https://doi.org/10.1007/s10973-020-10431-5.
Abbas-Abadi MS, Van Geem KM, Fathi M, Bazgir H, Ghadiri M. The pyrolysis of oak with polyethylene, polypropylene and polystyrene using fixed bed and stirred reactors and TGA instrument. Energy. 2021;232:121085.
Ryu HW, Kim DH, Jae J, Lam SS, Park ED, Park Y-K. Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons. Biores Technol. 2020;310:123473.
Abbas-Abadi MS, Jalali A, Rostami MR, Haghighi MN, Farhadi A. The atmospheric, vacuum and pressurized pyrolysis of used bleaching soils along with polymeric wastes to reach the valuable and economical fuels. J Clean Prod. 2020;255:120328. https://doi.org/10.1016/j.jclepro.2020.120328.
Seifali Abbas-Abadi M, Nekoomanesh HM. The consideration of different effective zeolite based catalysts and heating rate on the pyrolysis of Styrene Butadiene Rubber (SBR) in a stirred reactor. Energy Fuels. 2017;31(11):12358–63.
Bech N, Jensen PA, Dam-Johansen K. Determining the elemental composition of fuels by bomb calorimetry and the inverse correlation of HHV with elemental composition. Biomass Bioenerg. 2009;33(3):534–7.
Quek A, Balasubramanian R. Liquefaction of waste tires by pyrolysis for oil and chemicals—a review. J Anal Appl Pyrol. 2013;101:1–16.
Gao Y, Zheng B, Wu G, Ma F, Liu C. Effect of the Si/Al ratio on the performance of hierarchical ZSM-5 zeolites for methanol aromatization. RSC Adv. 2016;6(87):83581–8.
Önal E, Uzun BB, Pütün AE. Bio-oil production via co-pyrolysis of almond shell as biomass and high density polyethylene. Energy Convers Manage. 2014;78:704–10.
Muniandy L, Adam F, Mohamed AR, Ng E-P. The synthesis and characterization of high purity mixed microporous/mesoporous activated carbon from rice husk using chemical activation with NaOH and KOH. Microporous Mesoporous Mater. 2014;197:316–23.
Shagali AA, Hu S, Wang Y, Li H, Wang Y, Su S, et al. Comparative study on one-step pyrolysis activation of walnut shells to biochar at different heating rates. Energy Rep. 2021;7:388–96.
Bedia J, Peñas-Garzón M, Gómez-Avilés A, Rodriguez JJ, Belver C. Review on activated carbons by chemical activation with FeCl3. C. 2020;6(2):21.
Alfattani R, Shah MA, Siddiqui MIH, Ali MA, Alnaser IA. Bio-char characterization produced from walnut shell biomass through slow pyrolysis: sustainable for soil amendment and an alternate bio-fuel. Energies. 2021;15(1):1.
Abbas-Abadi MS, Haghighi MN, Yeganeh H, Bozorgi B. The effect of melt flow index, melt flow rate, and particle size on the thermal degradation of commercial high density polyethylene powder. J Therm Anal Calorim. 2013;114(3):1333–9.
Brebu M, Ucar S, Vasile C, Yanik J. Co-pyrolysis of pine cone with synthetic polymers. Fuel. 2010;89(8):1911–8.
Abbas-Abadi MS, Haghighi MN, Yeganeh H. Effect of the melt flow index and melt flow rate on the thermal degradation kinetics of commercial polyolefins. J Appl Polym Sci. 2012;126(5):1739–45.
Ding Y, Ezekoye OA, Lu S, Wang C, Zhou R. Comparative pyrolysis behaviors and reaction mechanisms of hardwood and softwood. Energy Convers Manage. 2017;132:102–9.
Greenhalf C, Nowakowski D, Harms A, Titiloye J, Bridgwater A. A comparative study of straw, perennial grasses and hardwoods in terms of fast pyrolysis products. Fuel. 2013;108:216–30.
Burhenne L, Messmer J, Aicher T, Laborie M-P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J Anal Appl Pyrol. 2013;101:177–84.
Han B, Chen Y, Wu Y, Hua D, Chen Z, Feng W, et al. Co-pyrolysis behaviors and kinetics of plastics–biomass blends through thermogravimetric analysis. J Therm Anal Calorim. 2014;115(1):227–35.
Ahmed MB, Johir MAH, Zhou JL, Ngo HH, Nghiem LD, Richardson C, et al. Activated carbon preparation from biomass feedstock: clean production and carbon dioxide adsorption. J Clean Prod. 2019;225:405–13.
Chen W, Gong M, Li K, Xia M, Chen Z, Xiao H, et al. Insight into KOH activation mechanism during biomass pyrolysis: chemical reactions between O-containing groups and KOH. Appl Energy. 2020;278:115730.
Abbas-Abadi MS, Zayoud A, Kusenberg M, Roosen M, Vermeire F, Yazdani P, et al. Thermochemical recycling of end-of-life and virgin HDPE: a pilot-scale study. J Anal Appl Pyrol. 2022;166:105614.
Abbas-Abadi MS, Ureel Y, Eschenbacher A, Vermeire FH, Varghese RJ, Oenema J, et al. Challenges and opportunities of light olefin production via thermal and catalytic pyrolysis of end-of-life polyolefins: towards full recyclability. Progr Energy Combust Sci. 2023;96:101046. https://doi.org/10.1016/j.pecs.2022.101046.
Abbas-Abadi MS, Kusenberg M, Zayoud A, Roosen M, Vermeire F, Madanikashani S, et al. Thermal pyrolysis of waste versus virgin polyolefin feedstocks: the role of pressure, temperature and waste composition. Waste Manage. 2023;165:108–18. https://doi.org/10.1016/j.wasman.2023.04.029.
Acknowledgements
The research leading to these results has also received funding from the Fund for Scientific Research Flanders (FWO) and innovation program/ERC grant agreement no.818607 (OPTIMA). This work was also performed in the framework of the Catalisti cluster SBO project WATCH (HBC.2019.0001 “Plastic waste to chemicals”) and PREFER (The Plastics Refinery: No More Waste) with the financial support of VLAIO (Flemish Agency for Innovation and Entrepreneurship).
Author information
Authors and Affiliations
Contributions
HB helped in methodology, software, validation, and writing—review & editing; MRR contributed to conceptualization, software, and writing—original draft; ST, BG and ZI helped in software, writing—original draft; HMS contributed to methodology and validation; KMVG done methodology, validation, writing—review & editing, and supervision; MNH performed conceptualization, writing—original draft, writing—review & editing; MSA-A contributed to conceptualization, software, and writing—original draft.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bazgir, H., Rostami, M.R., Tavakkol, S. et al. The chemical process of producing activated carbon using walnut shells and plastic wastes. J Therm Anal Calorim 148, 10125–10138 (2023). https://doi.org/10.1007/s10973-023-12364-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12364-1