Abstract
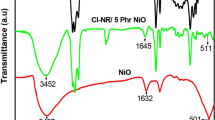
The manufacture of flexible devices is of immense interest due to their eco-friendliness, economic prospects and wide range of applications in biocompatible devices. Therefore, this paper reports the characterization and properties of chlorinated natural rubber (Cl-NR)/polyindole (PIN) blend with different contents of nickel oxide (NiO) nanoparticles. The formation of blend nanocomposites was analysed by FT-IR, UV spectroscopy, XRD, FE-SEM, DSC and TGA. The FT-IR and UV spectra proved the effective interfacial interaction between nanoparticles and the blend matrix, with a shift in UV peak intensity with the attachment of NiO at 433 cm−1. The XRD pattern revealed the regular arrangement of the blend matrix due to the presence of nanofiller, resulting in the semicrystalline structure of the composite system. The SEM micrographs revealed a homogeneous distribution of NiO in the blend matrix. The increased degradation temperature in the TGA demonstrated the enhanced thermal stability of the NiO-filled Cl-NR/PIN blend. DSC results showed that the glass transition temperature of the composite increased with NiO content in the blend matrix. The dielectric properties and AC electrical conductivity increased significantly with increasing temperatures, as well as with the addition of nanoparticles up to 5 mass % loading. The inclusion of NiO improved the tensile strength and hardness while reducing the elongation-at-break of nanocomposites. The blend nanocomposite with enhanced thermal stability, glass transition temperature, tensile strength, AC conductivity and dielectric properties enables them to be used in highly flexible electronic applications where traditional elastomeric materials fall short.










Similar content being viewed by others
References
Amorim DRB, Bellucci FS, Job AE, Guimaraes IS, Cunha HN. Electrical, structural and thermal properties of new conductive blends (PANICG) based on polyaniline and cashew gum for organic electronic. J Therm Anal Calorim. 2019;136:1615–29. https://doi.org/10.1007/s10973-018-7778-6.
Yamani K, Berenguer R, Benyoucef A, Morallon E. Preparation of polypyrrole (PPy)-derived polymer/ZrO2 nanocomposites. J Therm Anal Calorim. 2019;135:2089–100. https://doi.org/10.1007/s10973-018-7347-z.
Sofiah AG, Samykano M, Shahabuddin S, Kadirgama K, Pandey AK. An experimental study on characterization and properties of eco-friendly nanolubricant containing polyaniline (PANI) nanotubes blended in RBD palm olein oil. J Therm Anal Calorim. 2021;145:2967–81. https://doi.org/10.1007/s10973-020-09891-6.
Suvarna S, Furhan, Ramesan MT. (2023) Structural, conductivity, mechanical and wettability properties of copper alumina reinforced chlorinated polyethylene/polyvinyl chloride blend nanocomposites. Res Chem Intermed. 49(5):1891-908. https://doi.org/10.1007/s11164-022-04881-9
Camillo EC, Constantino CJ, Teruya MY, Alves N, Mattoso LH, Job AE. Dependence of the electrical conductivity and elastomeric properties on sample preparation of blends of polyaniline and natural rubber. J Appl Polym Sci. 2005;97:1498–503. https://doi.org/10.1002/app.21899.
De Paoli MA, Gazotti WA. Conductive polymer blends: preparation, properties and applications. Macromol Symposia. 2002;189:83–104. https://doi.org/10.1002/masy.200290008.
Silva MJ, Soares VO, Dias GC, Santos RJ, Job AE, Sanches AO, Malmonge JA. Study of thermal and mechanical properties of a biocomposite based on natural rubber and 45S5 Bioglass® particles. J Therm Anal Calorim. 2018;131:735–42. https://doi.org/10.1007/s10973-016-5933-5.
Masek A, Diakowska K, Zaborski M. Physico-mechanical and thermal properties of epoxidized natural rubber/polylactide (ENR/PLA) composites reinforced with lignocellulose. J Therm Anal Calorim. 2016;125:1467–76. https://doi.org/10.1007/s10973-016-5682-5.
Nghia PT, Onoe H, Yamamoto Y, Kawahara S. Hydrogenation of natural rubber having epoxy group. Colloid Polym Sci. 2008;286:993–8. https://doi.org/10.1007/s00396-008-1859-1.
Gnecco S, Pooley A, Lefimil C, Pino C, Valenzuela L. Chlorination of low-molecular-weight Euphorbia lactiflua natural rubber. Polym Bull. 1997;39:605–12. https://doi.org/10.1007/s002890050192.
Suvarna S, Furhan, Parvathi K, Ramesan MT. (2023) Role of copper alumina nanoparticles on the performance of polyvinylchloride nanocomposites. J Vinyl Addt Technol. 29: 17–28. https://doi.org/10.1002/vnl.21939
Ramesan MT, Suvarna S. Effect of Cu-Al2O3 nanoparticles on the performance of chlorinated polyethylene nanocomposites. Prog Rubber Plast Recycl Technol. 2023;31:81–95. https://doi.org/10.1177/14777606221136152.
Wang W, Ren G, Wang M, Liu Y, Wu S, Shen J. A novel composite for energy storage devices: core–shell MnO2/polyindole nanotubes supported on reduced graphene oxides. J Mater Sci Mater Electron. 2018;29:5548–60. https://doi.org/10.1007/s10854-018-8523-4.
Mudila H, Prasher P, Kumar M, Kumar A, Zaidi MG, Kumar A. Critical analysis of polyindole and its composites in supercapacitor application. Mater Renew Sustain Energy. 2019;8:9. https://doi.org/10.1007/s40243-019-0149-9.
Zhu D, Zhou Q, Liang A, Zhou W, Chang Y, Li D, Wu J, Ye G, Xu J, Ren Y. Two-step preparation of carbon nanotubes/RuO2/polyindole ternary nanocomposites and their application as high-performance supercapacitors. Front Mater Sci. 2020;14:109–19. https://doi.org/10.1007/s11706-020-0497-5.
Jiajia W, Zhanwen D, Ping X, Zhijiang C. Fabrication of flexible polyindole/carbon nanotube/bacterial cellulose nanofiber nonwoven electrode doped by D-tartaric acid with high electrochemical performance. Cellulose. 2020;27:6353–66. https://doi.org/10.1007/s10570-020-03199-2.
Khati K, Joshi I, Bisht A, Zaidi MG. Haemoglobin/polyindole composites: the novel material for electrochemical supercapacitors. Bull Mater Sci. 2019;42:20. https://doi.org/10.1007/s12034-018-1700-5.
Zhou Q, Zhu D, Ma X, Xu J, Zhou W, Zhao F. High-performance capacitive behavior of layered reduced graphene oxide and polyindole nanocomposite materials. RSC Adv. 2016;6:29840–7. https://doi.org/10.1039/c5ra27375g.
Hashemi SA, Naderi HR, Mousavi SM, Bahrani S, Arjmand M, Dimiev AM, Ramakrishna S. Synergic effect of laser-assisted graphene with silver nanowire reinforced polyindole/polypyrrole toward superior energy density. Carbon. 2022;188:276–8. https://doi.org/10.1016/j.carbon.2021.12.028.
Anjitha T, Anilkumar T, Mathew G, Ramesan MT. Zinc ferrite @ polyindole nanocomposites: synthesis, characterization and gas sensing applications. Polym Compos. 2019;40:2802–11. https://doi.org/10.1002/pc.25088.
Lee H, Park SC, Roh JS, Moon GH, Shin JE, Kang YS, Park HB. Metal–organic frameworks grown on a porous planar template with an exceptionally high surface area: promising nanofiller platforms for CO 2 separation. J Mater Chem A. 2017;5:22500–5.
Zhang H, Cao D, Jiang Z, Yu H, Shi A, Bai X. Trunk-Leaf Vein” structure inspired synthesis of mesoporous carbon @ nickel oxide/nickel ternary composite for sustainable supercapacitor electrode. Appl Surf Sci. 2022;571:151324. https://doi.org/10.1016/j.apsusc.2021.151324.
Parvathi K, Ramesan MT. High performance chlorinated natural rubber/ zinc ferrite nanocomposite prepared through industrial compounding technique. Polym Bull. 2023;80:3165–82. https://doi.org/10.1007/s00289-022-04201-6.
Talbi H, Ghanbaja J, Billaud D, Humbert B. Vibrational properties and structural studies of doped and dedoped polyindole by FTIR. Raman EEL Spect, Polym. 1997;38:2099–106. https://doi.org/10.1016/S0032-3861(96)00759-8.
Rolere S, Liengprayoon S, Vaysse L, Sainte-Beuve J, Bonfils F. Investigating natural rubber composition with fourier transform infrared (FT-IR) spectroscopy: a rapid and non-destructive method to determine both protein and lipid contents simultaneously. Polym Test. 2015;43:83–93. https://doi.org/10.1016/j.polymertesting.2015.02.011.
Parvathi K, Ramesan MT. Insights into structural, thermal, electrical and mechanical properties of copper alumina reinforced chlorinated natural rubber nanocomposites. J Thermoplast Compos Mater. 2022. https://doi.org/10.1177/08927057221083495.
Parvathi K, Ramesan MT. Structure, properties and antibacterial behaviour of nickel oxide reinforced natural rubber nanocomposites for flexible electronic applications. J Appl Polym Sci. 2022;139:e53120. https://doi.org/10.1002/app.53120.
Saikia JP, Paul S, Konwar BK, Samdarshi SK. Nickel oxide nanoparticles: a novel antioxidant. Colloids Surf, B. 2010;78:146–8. https://doi.org/10.1016/j.colsurfb.2010.02.016.
Kumar YR, Deshmukh K, Naseer Ali MM, Abhijay G, Al-Onazi WA, Al-Mohaimeed AM, Khadheer PSK. Structure, morphology and modelling studies of poly vinyl alcohol nanocomposites reinforced with nickel oxide nanoparticles and graphene quantum dots. Environ Res. 2022;203:111842. https://doi.org/10.1016/j.envres.2021.111842.
Holzwarth U, Gibson N. The Scherrer equation versus the ‘Debye-Scherrer equation.’ Nature Nanotech. 2011;6:534. https://doi.org/10.1038/nnano.2011.145.
Khalil AM, Rabie ST. Mechanical, thermal and antibacterial performances of acrylonitrile butadiene rubber/polyvinyl chloride loaded with Moringa oleifera leaves powder. J Therm Anal Calorim. 2021;143:2973–81. https://doi.org/10.1007/s10973-019-09194-5.
Palaniappan S, John A. Facile synthesis of bis (indolyl) methanes using polyindole salt as reusable catalyst. J Mol Catal A Chem. 2005;242:168–72. https://doi.org/10.1016/j.molcata.2005.07.041.
Sankar S, Ramesan MT. Thermal, optical and temperature-dependent electrical properties of poly(aniline-co-pyrrole)/copper alumina nanocomposites for optoelectronic devices. J Therm Anal Calorim. 2022;147:13375–87. https://doi.org/10.1007/s10973-022-11670-4.
Mi HY, Li Z, Turng LS, Sun Y, Gong S. Silver nanowire/thermoplastic polyurethane elastomer nanocomposites: thermal, mechanical, and dielectric properties. Mater Design. 2014;56:398–404. https://doi.org/10.1016/j.matdes.2013.11.029.
Pan C, Markvicka EJ, Malakooti MH, Yan J, Hu L, Matyjaszewski K, Majidi C. A liquid-metal–elastomer nanocomposite for stretchable dielectric materials. Adv Mater. 2019;31:1900663. https://doi.org/10.1002/adma.201900663.
Bhagat DJ, Dhokane GR. Novel synthesis and DC electrical studies of polyindole/poly(vinyl acetate) composite films. Chem Phys Lett. 2015;619:27–31. https://doi.org/10.1016/j.cplett.2014.11.052.
Zhu L. Exploring strategies for high dielectric constant and low loss polymer dielectrics. J Phys Chem Lett. 2014;5:3677–87. https://doi.org/10.1021/jz501831q.
Liu R, Wang J, Li Q, Li S, Zhang S, Ding X. Copper phthalocyanine oligomer grafted acrylic elastomer nanocomposites with high dielectric constants. J Appl Polym Sci. 2014. https://doi.org/10.1002/app.39975.
Saji J, Khare A, Choudhary R, Mahapatra S. Impedance analysis, dielectric relaxation, and electrical conductivity of multi-walled carbon nanotube-reinforced silicon elastomer nanocomposites. J Elastomers Plast. 2015;47:394–415. https://doi.org/10.1177/0095244313514991.
Dutta K, De SK. Electrical conductivity and dielectric properties of SiO2 nanoparticles dispersed in conducting polymer matrix. J Nanopart Res. 2007;9:631–8. https://doi.org/10.1007/s11051-006-9184-4.
Jegadheeswaran S, Sundaramahalingam A, Pohekar SD. High-conductivity nanomaterials for enhancing thermal performance of latent heat thermal energy storage systems. J Therm Anal Calorim. 2019;138:1137–66. https://doi.org/10.1007/s10973-019-08297-3.
Ramesan MT, Jayakrishnan P, Sampreeth T, Pradyumnan PP. Temperature-dependent AC electrical conductivity, thermal stability and different DC conductivity modelling of novel poly(vinyl cinnamate)/zinc oxide nanocomposites. J Therm Anal Calorim. 2017;129:135–45. https://doi.org/10.1007/s10973-017-6140-8.
Meera K, Ramesan MT. Performance of boehmite nanoparticles reinforced carboxymethyl chitosan/polyvinyl alcohol blend nanocomposites tailored through green synthesis. J Polym Environ. 2023;31:447–60. https://doi.org/10.1007/s10924-022-02649-1.
Kumar V, Alam MN, Park SS. Robust magneto-rheological elastomers performance for composites based on iron oxide and carbon black in silicone rubber. J Polym Res. 2022;29:251.
Acknowledgements
The authors greatly acknowledge the financial assistance from KSCSTE, Government of Kerala, India (Order No.566/2017/KSCSTE).
Funding
Funding was provided by Kerala State Council for Science, Technology and Environment, 566/2017 KSCSTE, M. T. Ramesan
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parvathi, K., Verma, M. & Ramesan, M.T. Enhanced thermal, electrical and mechanical properties of nickel oxide reinforced chlorinated natural rubber/poly (indole) blend nanocomposites. J Therm Anal Calorim 148, 10139–10149 (2023). https://doi.org/10.1007/s10973-023-12358-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12358-z