Abstract
The overall effect of reactants in the form of sulfates of ammonium, calcium, copper(II), iron(III), manganese(II) and zinc on the thermal behavior of ammonium nitrate has been reported. Thermal stability assessment was performed with the use of thermogravimetric analysis and differential thermal analysis coupled with mass spectroscopy. Interestingly, sulfate anions present in the system were often not sufficient to properly inhibit the decomposition of ammonium nitrate. Sulfate ion and cation supplied with the selected compound significantly influence the studied process. Studied mixtures with ammonium sulfate and calcium sulfate were concluded to show the highest stability. Manganese and iron sulfate salts caused a significant acceleration of the initial thermal decomposition. The addition of these compounds led to visible changes in the process mechanism, which allowed them to be classified as catalysts of the decomposition of ammonium nitrate. Furthermore, small amounts of substances in the system, even those that are generally considered to be inhibitors, worsened the thermal stability of AN. Zinc and copper sulfates, under studied conditions, created double salts that were characterized by a higher thermal stability than pure ammonium nitrate. This property indicates the possibility of obtaining systems containing ammonium nitrate with significantly higher thermal stability, what could potentially have multiple useful applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ammonium nitrate (AN) has found applications in various areas of the chemical industry because of its physicochemical properties and thermal characteristics. Specifically, it has been used as an oxidizer or as a chloride-free solid propellant [1, 2]. Compositions based on ammonium nitrate also find special use in mining and military industries as explosives [3, 4]. Moreover, due to the characteristics of its thermal decomposition and complete decomposition into gaseous products in the form of nitrogen compounds, ammonium nitrate has been considered as a potential solid compound to form matrices of solid, environmentally friendly propellants [5]. Its suggested application might be the use of phase-stabilized ammonium nitrate (PSAN) mixtures to generate the gas phase in airbag systems [6]. Most importantly, due to its high nitrogen content in forms readily available to plants, ammonium nitrate-based products are considered as attractive and economically viable compositions in the fertilizer industry [7]. Popular compositions listed mineral nitrogen products as solid fertilizers such as calcium ammonium nitrate (CAN) and nitrogen-phosphorus-potassium (NPK) systems or liquid urea-ammonium nitrate (UAN) systems [8, 9].
Products based on ammonium nitrate contain numerous additives in order to obtain appropriate physicochemical properties toward the final application. One of the most important features involves the evaluation of the effect of inhibiting, catalyzing or dissipating the thermal decomposition of ammonium nitrate by the introduced additive. The most common promoters of AN decomposition include Cl− ions, transition metal compounds, mineral acids and most of organic compounds [5, 10,11,12]. The effect of delaying (inhibiting) AN decomposition can be expected from compounds with alkaline properties [2, 13, 14]. Calcium carbonate (CaCO3) is considered as one of the most effective inhibitors of AN decomposition [2, 9, 15]. Additives introduced into AN can also impact the system by causing the dissipation of energy released by the thermal decomposition of AN. These compounds have been classified as inert toward AN, with many examples, such as (NH4)2SO4, CaSO4, being defined in the literature [9, 16]. However, the impacts of some compounds on the course of the thermal decomposition of AN might be complicated to define, hence it can be difficult to estimate how a given additive affects thermophysical properties of ammonium nitrate systems. Oxley et al. [2] have done extensive research on evaluating the effects of many chemical compounds on the thermal stability of AN. Among the typical promoters and inhibitors of the AN decomposition, they also found compounds that behaved both as inhibitors and promoters, depending on their mass proportion in the mixture. Reported results suggest that, e.g., a 5 mass% addition of Ca(NO3)2 or Ca(HSO4)2 shifted the decomposition of AN to the higher temperature range, while as much as 20 mass% of their addition caused a visible acceleration of the decomposition process. Han et al. [17] showed that the shift of the thermal decomposition toward higher temperatures of the AN – Na2SO4 mixture increases with the higher contribution of the introduced additive. Nevertheless, the results also indicated that the rate of temperature and pressure rise in the system were highest for the sample with a 1.1 mass% of Na2SO4. Nowadays, it is accepted that the thermal stability of systems containing ammonium nitrate can vary for the same compositions that are studied under different conditions, even to the extent of obtaining either endo- or exothermic decomposition processes.
Another equally important aspect is related to the polymorphism of AN — the phase stabilization caused by introduced additives [18, 19]. That treatment removes crystallographic transformations of AN, so that granulated ammonium nitrate-based products retain their mechanical properties [20, 21]. Furthermore, selected additives decide the shape, durability and porosity of produced granules [22, 23]. The high hygroscopicity of AN-based products requires the necessary use of a wide range of additives with anticaking abilities [24]. Among them are mainly organic compounds, amine and paraffin mixtures, as well as polymers, aimed at forming a hydrophobic layer on the surface of AN-based products [25].
In the fertilizer industry, additives used in the production process, in addition to affecting the physicochemical properties of the final fertilizer composition, provide a source of macro- and micronutrients for plants. Recently, a lot of attention has been paid to mixtures with micronutrient compounds. Among them are compounds of boron, cobalt, copper, iron, manganese, molybdenum and zinc [26, 27]. All of them participate in many biochemical processes of plants, and their deficiency in the environment has a negative impact on crop quality and yield [26, 28, 29]. Maintaining an optimal concentration of micronutrients in the soil also protects plants from biotic and abiotic stress [30, 31].
The perspective of increased interest and growing market related to the necessity of supplementing deficiencies of macro- and microelements in the environment gives incentives to undertake this work. The issue associated with characteristics of structures that form in multicomponent ammonium nitrate-based fertilizer systems, due to the most recent explosion accidents in Tianjin [32] and Beirut [33], is a main factor increasing the need to conduct further research on the safety assessment and evaluation of handling, production and storage of above-mentioned products. This work aims at presenting a study of the effects of sulfates, previously classified as promoters, inhibitors or inerts, on the thermal properties of ammonium nitrate, under limited mass transfer conditions to ensure maximization of exothermic effects of the sample decomposition. Such conditions are best suited to simulate hazardous simulations that often cause AN disasters. Because of that, results presented in this manuscript are, in most cases, completely novel and, as such, advance the understanding of the thermal stability of AN systems containing sulfate salts, which can be used in future studies of fertilizer formulations containing sulfur and/or microelements.
Materials and methods
Materials
For the preparation of all mixtures, only high-quality components were used. Ammonium nitrate pure p.a. (EUROCHEM, Poland), ammonium sulfate pure p.a. (POCH, Poland), calcium sulfate hemihydrate pure p.a. (POCH, Poland), copper(II) sulfate pentahydrate pure p.a. (CHEMPUR, Poland), iron(III) sulfate hydrate pure p.a. (CHEMPUR, Poland), manganese(II) sulfate monohydrate pure p.a. (POCH, Poland) and zinc sulfate heptahydrate pure p.a. (POCH, Poland) were used without further purification or drying.
Methods
Sample preparation
Every mixture was prepared to contain ammonium nitrate with other sulfate salts in the mass ratio of 4:1, 9:1 and 49:1. To obtain homogeneous mixtures, components were ground in agate mortal for around 5 min and weighed in the alumina crucible afterward. The final mass of the mixture was correlated with the mixture ratio; thus, in each measurement, the mass of AN in the sample was equal to 20 mg. To better visualize the effect of each additive on the thermal stability of AN, DTA-TG measurement of pure ammonium nitrate (20 mg) and high-temperature DSC-TG measurements of pure sulfate salts (10 mg) were also performed.
Thermogravimetric analysis (TG) and differential thermal analysis (DTA) coupled with mass spectroscopy (MS)
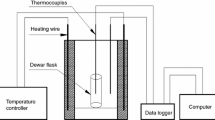
Thermal stability assessment was performed by evaluation of the thermal analysis run provided by thermogravimetric analysis (TG) and differential thermal analysis (DTA) coupled with mass spectroscopy (MS). For TG–DTA and MS analysis, STA 449 F3 Jupiter (Netzsch, Germany) and QMS Aeolos 403 C (Netzsch, Germany) were used, respectively. The thermal program includes a heating rate equal to 5 K·min−1 to a temperature of 400 °C with synthetic air flow of 30 mL·min−1. The sample was inserted into an alumina (Al2O3) crucible with a pierced lid. Each experiment was repeated three times. Evolved gas analysis performed by MS identity peak mass-to-charge ratio contained following signals: 15 (NH3), 17 (NH3, H2O), 18 (H2O), 30 (NO, NO2, N2O), 44 (N2O), 46 (NO2) and 64 (SO2). The appropriate methodology has been selected according to the reasoning given in previous work by the research group [34].
Results and discussion
Ammonium nitrate
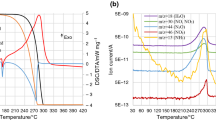
Results obtained during the DTA-TG analysis of pure ammonium nitrate are shown in Fig. 1. The exothermic decomposition process of ammonium nitrate has already been defined in the previous work of the research group, together with the broad explanation of parameters chosen to assess thermal stabilities of studied samples [34]. The only significant difference was the temperature of the beginning of the decomposition process, defined as the temperature at which gaseous products of the process were first detected and the rate of the mass loss achieved the value of 1% of AN contained in the sample per minute. All characteristic values used for the definition of thermal stability are presented in Table 1, and values for the change in mass of every sample in successive stages of the decomposition process are shown in Table 2.
Ammonium sulfate
The decomposition process of the ammonium sulfate sample is shown in Fig. 2. The total mass decrease was above 95%. The residue is suspected to contain impurities that were present in the pure reagent. Observed results suggest two stages of the decomposition of ammonium sulfate. Above 250 °C, it has undergone an initial decomposition, leading to a suspected formation of ammonium pyrosulfate [35, 36] which further decomposed at higher temperatures into gaseous products in the temperature range from 330 to 420 °C.
Figure 3 shows results of the TG–DTA–MS analysis of mixtures of ammonium nitrate and ammonium sulfate. Several subsequent mass steps can be observed when the mixture is heated to 400 °C. The first step strongly corresponds to the exothermic decomposition reaction of ammonium nitrate and two visible mass losses occur above 300 °C. At this point, a slow decomposition of ammonium sulfate may be observed, as defined for the pure compound.
The decomposition of ammonium sulfate occurs very slowly; therefore, in the system containing the most ammonium sulfate (4:1 ratio), a mass loss is still visible under measurement conditions. However, in systems where the addition of ammonium sulfate was significantly smaller, a complete decomposition of the sample could be observed before reaching 400 °C. In addition, neither amount of the introduced additive had a significant effect on phase transition temperatures of ammonium nitrate. During thermal decomposition, NH3, H2O, NO, N2O, NO2 and SO2 appear in the gas phase around 280 °C. At temperatures above 330 °C, the formation of NH3, SO2 and H2O was observed, as well as a subtle peak suggestive of a trace presence of NO2. Ammonium sulfate has been previously classified as an inhibitor of ammonium nitrate decomposition [2]. However, the study shows that this property is retained when the proportion of ammonium sulfate in the mixture is high. In the 4:1 system, the beginning of thermal decomposition takes place at a temperature of 237.0 °C, while in 9:1 and 49:1 systems, this temperature was 236.0 and 233.9 °C, respectively. It is suggested that, in small quantities, the addition of ammonium sulfate to ammonium nitrate could have a catalytic effect. In addition, the catalytic effect of small amounts of ammonium sulfate in the ammonium nitrate system is evident in the obtained DTA curve, where the maximum of the exothermic decomposition in the 49:1 system is seen at 281.2 °C and in the 9:1 and 4:1 systems at 289.3 and 287.1 °C, respectively. This can be explained with the formation of ions that are active in the autocatalytic decomposition of ammonium nitrate. According to the ionic decomposition of ammonium nitrate, ions such as HSO −4 or SO 2−4 , created during the first stage of the decomposition of ammonium sulfate, could be an additional reactant that further acidifies the sample [37]. Moreover, in the semi-closed system, the mass transfer between the crucible and the furnace is limited. The additional amount of NH3 from the decomposition of (NH4)2SO4 could shift the equilibrium in the AN dissociation reaction. Furthermore, the presence of NH +4 , SO 2−4 , HSO −4 and H2SO4 in the high-temperature AN system could increase the homolysis of nitric acid. The cumulation of these factors may result in an acceleration of the observed decomposition of ammonium nitrate. What is more, with increasing temperature, ammonium nitrate decomposition is supposed to become dominated by a radical mechanism [38]. On the other hand, the accumulation of HSO −4 successfully reduces the amount of free radicals (OH· and NO2·), resulting in a slowing of decomposition. On the basis of obtained results, one could conclude that, in the system with the lower amount of ammonium sulfate, described processes are difficult to distinguish. The presence of H2SO4 and other compounds from the thermal decomposition of ammonium sulfate probably catalyzes the decomposition of ammonium nitrate. When the amount of ammonium sulfate is higher, the dissociation of AN is limited by the created ammonia and further exothermic decomposition steps are inhibited.
Calcium sulfate hemihydrate
Figure 4 presents the dehydration of calcium sulfate hemihydrate. The main dehydration process occurred until the temperature of 200 °C was reached and the final residual mass fluctuated between 96.5 and 96.0%. Under thermal treatment, approximately 0.3 mol of water, out of 0.5, were evaporated from the hydrated calcium sulfate sample. When the partial dehydration of this compound was observed in reported temperature ranges, it could have been assumed that the interaction between almost anhydrous calcium sulfate and ammonium nitrate has been studied in this work.
The decomposition of the mixture of ammonium nitrate and calcium sulfate is shown in Fig. 5. Several scientific publications have examined these systems [2, 9]. Studies of systems with varying additive mass ratios, performed in this work, suggest that the decomposition of ammonium nitrate was more rapid when the percentage of calcium sulfate was at its lowest, contrary to the common concept of an undisputable inhibiting effect of this additive. The decomposition became slower with increasing additive proportion, resulting in thermal decomposition starting points of 236.7, 234.0 and 233.3 °C in 4:1, 9:1 and 49:1 systems, respectively. This is in agreement with previous studies in which calcium sulfate was classified as an inhibitor or inert substance of the ammonium nitrate decomposition. A reaction took place in the tested mixture, resulting in a creation of small amounts of calcium nitrate and ammonium sulfate. The appearance of ammonium sulfate in the reaction system can be associated with its progressive decomposition and the formation of reactive radicals that are involved in the exothermic effect of the ammonium nitrate decomposition reaction. Also, phenomena described in the previous section about the impact of a small amount of (NH4)2SO4 during the AN decomposition might have occurred. This event explains the pseudocatalytic effect that occurs when a small percentage of calcium sulfate is used as part of the mixture. A different effect is observed when the proportion of calcium sulfate in the system increases. This has the effect of prolonging and slowing down decomposition reactions. Perhaps, larger proportions of calcium sulfate in the system provide almost inert mediums to disperse the released energy; hence, the decomposition temperature range is very wide and the amount of total heat released tends to increase as the ratio of the additive in the mixture decreases. During the thermal decomposition of these mixtures, NH3, H2O, NO, N2O and NO2 were detected in the gas phase. At a temperature of approximately 290 °C, SO2 was recorded. This phenomenon indicates that a reaction took place between the components and a partial conversion to ammonium sulfate and calcium nitrate took place.
Copper(II) sulfate pentahydrate
Results of the thermal analysis of the hydrated copper sulfate are shown in Fig. 6. Three stages of sample dehydration can be seen with well-defined mass effects. The first one was observed up to around 81 °C. The next stage started almost immediately at approximately 85 °C and continued up to 119 °C. The mass loss in these stages was equal to 14.5 and 13.2%, respectively. The third mass loss started at 190 °C and ended at 255 °C, yielding a 6.9% decrease. The MS analysis showed only signals corresponding to H2O in gases created during the first three stages. The thermal decomposition path of hydrated copper sulfate was very similar to that previously described by other researchers in the literature [39]. During the first two steps, approximately four water molecules were released. In the last stage, the dehydration of the remaining water molecule was observed. The following two-step decomposition occurred in the range from 635 to 770 °C. It is suspected that CuSO4 · CuO is created as an intermediate product in the first step, followed by its further decomposition to copper oxide.
Figure 7 shows the thermal analysis and mass spectroscopy graphs of the mixture of ammonium nitrate and copper sulfate pentahydrate. Three successive steps of mass decrease were distinguished during the measurement. The mass loss observed up to approximately 175 °C suggests partial dehydration of the copper sulfate present in the mixture. Water contained in the compound can heavily influence phase transitions of ammonium nitrate, as a visible phase change III→II occurred. The next mass effect was mainly related to the exothermic decomposition of ammonium nitrate. The starting temperature of exothermic decomposition was equal to 229.2, 229.1 and 233.1 °C in 4:1, 9:1 and 49:1 systems, respectively. The value of the mass effect does not indicate the complete decomposition of ammonium nitrate. The rest of ammonium nitrate, or more precisely its decomposition products, could react with copper sulfate and form copper(II) tetraaminasulfate complex, which is considered stable under these conditions. The mass effect size of the third decomposition stage also points in this direction. However, the hypothesis that an equally stable CuSO4 · NH4NO3 double salt could be formed and decomposed separately above 300 °C is considered highly probable. It is believed that the ammonium nitrate reacted with copper sulfate, leading to the formation of a double salt or complex with ammonium ions formed during the thermal dissociation of the ammonium nitrate. No sulfur compounds were recorded in the decomposition products, hence the hypothesis that a compound comprised of ammonium nitrate and copper sulfate was formed, which effectively retards the thermal decomposition of ammonium nitrate. Furthermore, in the last decomposition step, NO2, N2O, NO and H2O were recorded in the gas products stream evacuated from the furnace. That indicates that the part of ammonium nitrate had to decompose, further proving the formation of some chemical structure that was thermally stable up to 300 °C. The computational calculation of this system, presented in the literature, suggests the interaction between this species and the formation of a small amount of copper(II) oxide and sulfur dioxide [40]. Performed studies have not proven this theory though, what may have been caused by non-stoichiometric ratios of salts contained in tested mixtures.
Iron(III) sulfate hydrate
The thermal decomposition of pure hydrated iron sulfate is shown in Fig. 8. Under the given temperature program, crystalline water was released from the sample over the heating to 325 °C. The mass decrease of 19% in total suggests that the tested iron sulfate was a pentahydrate. The main decomposition stage was recorded in the range of 560 – 700 °C. The supposed product of the decomposition should be Fe2O3 [41]; however, the observed mass loss was indicated to be only 55% of the mass of the anhydrous sulfate, while theoretical mass loss should be equal to almost 60%. Upon further heating, another small step was registered on the TG curve, adding the total mass loss of the anhydrous salt to 60% and confirming the theory of oxide formation.
The decomposition of ammonium nitrate mixtures with iron sulfate hydrate in mass ratios of 4:1, 9:1 and 49:1 is shown in Fig. 9. As the temperature increased, the appearance of three distinguished stages of decomposition was observed. The first stage was identified as the gradual dehydration of iron sulfate and lasted until 148.3, 153.9 and 157.5 °C, respectively. After these temperatures were reached, a rapid mass loss occurred up to approximately 200 °C. When the second step ended, the system underwent relative stabilization. Finally, the last mass step represented the catalyzed decomposition of ammonium nitrate. The beginning of the exothermic decomposition is estimated at temperatures of 220.0, 228.4 and 235.0 °C in 4:1, 9:1 and 49:1 systems, respectively. In contrast to systems containing copper sulfate, the process did not show the formation of a double salt or complex, unless it was decomposed along with ammonium nitrate in the same temperature range. Nevertheless, the formation of such combinations may not be assumed on the basis of obtained data. Phase transformations IV→III, IV→II, and II→I occurred at temperatures similar to those of the pure ammonium nitrate sample. The melting point of the mixture showed a significant difference. For the 4:1 and 9:1 systems, melting temperatures were 148.3 and 153.9 °C, respectively. In the 49:1 system, the peak associated with the melting of the mixture is broken into two endothermic maxima, whose temperatures are estimated at 157.5 and 165.1 °C. The low melting point of the mixture and the double peak in the system with the lowest iron sulfate content led to the conclusion that reactants possibly formed ammonium and iron sulfate in the studied system before the melting of the mixture. As the initial point of the beginning of the decomposition process of the 4:1 sample was at the lowest temperature among all studied mixtures, the iron salt should be considered as a catalyst of the decomposition process.
Manganese(II) sulfate monohydrate
The thermal decomposition of the hydrated manganese sulfate is presented in Fig. 10 and shows the dehydration process, which occurs in two distinct stages, and a decomposition of the compound. The total mass loss during dehydration was equal to 10.6%. In the first step of dehydration, which occurred in the temperature range from 115 to 170 °C, less than 20 mass% of total water was released. The second dehydration step lasted up to 265 °C, with the release of the rest of the bound water. The decomposition process was recorded in the range of 730 – 900 °C and resulted in a 42.2% of the total mass loss of the sample. Both the previous study of another research group [42] and obtained results support the claim that Mn2O3 was obtained as a product of this decomposition. In the range from 900 to approximately 950 °C, an additional mass loss was recorded and evaluated at around 1.3% of the total mass of the studied sample. This result was, again, in agreement with literature claims and allowed to conclude that the high-temperature formation of Mn3O4 occurred in the studied system.
The thermal decomposition of mixtures of ammonium nitrate with hydrated manganese sulfate is shown in Fig. 11. This decomposition process followed several stages. The first part lasted up to 210.6, 199.1 and 193.1 °C in 4:1, 9:1 and 49:1 samples, respectively. Above this temperature value, the rate of mass change gradually increased, leading to a strong and rapid decrease in 4:1 and 9:1 systems. The final stage was characterized by a linear and very slow decrease in mass, clearly visible in 4:1 and 9:1 compositions. The initiation of the exothermic decomposition was determined at temperatures of 237.3, 236.4 and 234.8 °C. The distinctive feature of the decomposition of ammonium nitrate mixed with the manganese salt is the intensive catalytic effect during the on-going decomposition of ammonium nitrate. Obtained exotherms are characterized by the sharp exothermic peak at temperatures of 292.4, 297.8 and 302.9 °C. However, at lower temperatures, an exothermic peak corresponding mainly to the uncatalyzed decomposition of ammonium nitrate is also visible. This type of thermal decomposition split suggests that the catalytic effect of manganese compounds depends on the stage of ammonium nitrate decomposition or becomes dominant at higher temperature values. Therefore, it is unnoticeable when the endothermic dissociation process of AN, which occurs during the initial phase of the decomposition process, is prevalent. The addition of manganese sulfate did not have a significant effect on phase transitions of ammonium nitrate. The heat effect of the exothermic decomposition process was dependent on the manganese sulfate content and was smaller the more sulfate salt was present in the system. It should be stated that even though the amount of generated heat was lower than that of the pure AN sample, the shape of the obtained exotherm and steepness of the TG curve strongly indicated the strong catalytic effect of the examined additive. During thermal decomposition, in addition to nitrogen oxides, ammonia and water, sulfur oxides were also observed in the gas phase. Since the decomposition of pure manganese sulfate monohydrate occurred above 700 °C, the presence of these signals indicated that a reaction between salts has taken place. Due to a possible reaction pathway, the decomposition dynamics of the mixture above 280 °C could be caused by the decomposition of the remaining ammonium nitrate and formed manganese(II) nitrate, which thermal decomposition is typically observed in the range of 280 – 300 °C. It can be suspected that the creation of manganese nitrate and its decomposition are highly catalytic radical reactions that accelerated the decomposition of AN.
Zinc sulfate heptahydrate
Hydrated zinc sulfate undergoes several stages of dehydration, with a clear sudden separation of some of the crystalline-bound water. From the TG curve shown in Fig. 12, three consecutive stages can be distinguished. Observed mass losses were equal to 7.5, 18.3 and 7.7%, respectively. According to calculations of changes in the mass of the sample, less water molecules were released in the subsequent stages than the literature or properties of the analyzed sample would suggest [43]. This may have been caused by the evaporation of water during the sample preparation and a stabilization of the thermal analyzer before the actual analysis has begun. The thermal decomposition of the anhydrous sulfate was recorded as a two-step process, what is also suggested in the literature, as a ZnO · 2ZnSO4 is obtained in the first step, before it further decomposes into zinc oxide. The observed decomposition process occurred in the range of 750 – 910 °C.
The TG–DTA–MS analysis of zinc sulfate and ammonium nitrate samples is presented in Fig. 13. These systems thermally decomposed in three well-separated mass steps. The first mass loss was due to the loss of crystalline water and occurred up to about 200 °C. This was confirmed by MS analysis, according to the peaks m/z = 17 and m/z = 18 identified with the H2O molecule. The increased amount of water in the system had an impact on the phase transition of ammonium nitrate; therefore, the transformation III→II, around 86 °C, was visible during the heating program. Depending on the proportion of zinc sulfate in the system, the initial value of the thermal decomposition temperature changed and rose with increasing zinc sulfate content. Next, the main decomposition of ammonium nitrate was observed and typical gas products, such as nitrogen oxides, ammonia and water, were recorded. A similar decomposition mechanism of the last stage, as observed in the decomposition of ammonium nitrate with copper sulfate, is suspected in this case. Based on the calculation from the experimental data, it is assumed that the mentioned double salt is characterized by the ratio of ZnSO4 · NH4NO3 that varies around 1.5 – 0.5. The ratio seemed to depend on the constitution of the prepared mixture. The assumption of such a hypothesis explains the existence of characteristic gases in the gas phase, associated with the decomposition of ammonium nitrate, with no sulfur dioxide signal recorded during the MS analysis. Obtained results indicate that zinc sulfate can be considered as either a neutral compound or a slight inhibitor in terms of the ammonium nitrate decomposition.
Conclusions
In this work, the effect on the thermal behavior of ammonium nitrate and a possible interaction of reactants, in the form of ammonium, calcium, copper, manganese, iron and zinc sulfates, has been reported. Most of these systems have never been studied before and the conditions used in this study allowed to properly define the thermal stability of said systems or verify some common misconceptions. It was observed that the presence of sulfate ions in the system had a positive effect in delaying the thermal decomposition of ammonium nitrate. In studied systems, a possible interaction of the reactants, leading to the appearance of additional portions of ammonia in the system during the initial phase of AN decomposition, was observed. This led to a change in the balance of the AN dissociation reaction, causing less nitric acid to be generated in the system and, therefore, the amount of homolysis products of HNO3, which are responsible for the radical mechanism of AN decomposition. Consequently, this moves the actual decomposition of AN to the higher temperature range.
In the case of the manganese sulfate system, Mn(NO3)2 is formed due to a partial reaction with AN and undergoes thermal decomposition at temperatures close to 300 °C. The parallel decomposition of both nitrates present in the system leads to a cumulative energy effect, significantly accelerating the thermal decomposition of the entire mixture. Calcium sulfate is typically considered an inert substance in systems with ammonium nitrate. Obtained results indicated the possibility of a partial reaction occurring between calcium sulfate and AN, similarly to the system containing manganese salt. Calcium sulfate was less reactive though and did not accelerate the ammonium nitrate decomposition to a similar extent as the manganese sulfate did.
Small amounts of any sulfate additive in the mixture with ammonium nitrate could be considered negative in terms of the assessed thermal stability, invalidating theories that certain sulfates should only be considered as inhibitors of the exothermic decomposition of ammonium nitrate. This issue is of a high importance, as an inappropriately defined influence of any additive may lead to potentially catastrophic consequences caused by unstable ammonium nitrate systems.
Iron sulfate did not have an obvious reaction pathway with ammonium nitrate at elevated temperatures. It visibly destabilized the mixture and has to be considered as a strong catalyst of the decomposition process.
The separated mass effect in systems with zinc sulfate and copper sulfate indicates that the most possible result was the formation of a double salt. Based on calculations, the ZnSO4 · NH4NO3 and CuSO4 · NH4NO3 ratios are changeable and range from 1.5 to 0.5 and 0.8 – 0.4, respectively. Values of these ratios may change significantly if the mass percentage of zinc or copper sulfate in the mixture increases. Double salts of zinc or copper sulfate with ammonium nitrate could be a potential carrier matrix for micronutrients, as their thermal stability seems to be relatively high. Surprisingly, copper sulfate was not defined as an obvious catalyst of the AN decomposition process, contrary to the popular belief that all copper compounds heavily destabilize ammonium nitrate.
The study does not exclude the possibility of the formation of other double-salt structures similar to those of copper or zinc. However, their presence did not visibly affect the general decomposition reaction of ammonium nitrate. Sulfate systems of double salts, containing ammonium nitrate, should be further investigated in terms of their thermal stability to determine the possibility of their use in the future.
References
Mousaviazar A, Keshavarz MH, Hayaty M. The effect of cellulose derivatives on the phase transition and thermal behavior of ammonium nitrate. J Therm Anal Calorim. 2017;128:1049–56. https://doi.org/10.1007/s10973-016-6031-4.
Oxley JC, Smith JL, Rogers E, Yu M. Ammonium nitrate: Thermal stability and explosivity modifiers. Thermochim Acta. 2002;384:23–45. https://doi.org/10.1016/S0040-6031(01)00775-4.
Oluwoye I, Altarawneh M, Gore J, Dlugogorski BZ. Burning properties of redox crystals of ammonium nitrate and saccharides. Combust Flame. 2020;213:132–9. https://doi.org/10.1016/j.combustflame.2019.11.030.
Wolny P, Tuśnio N, Mikołajczyk F. Explosion risks during firefighting operations in storage rooms and the transport of ammonium nitrate-based fertilizers. Sustainability. 2022;14:8565. https://doi.org/10.3390/su14148565.
Jos J, Mathew S. Ammonium nitrate as an eco-friendly oxidizer for composite solid propellants: promises and challenges. Crit Rev Solid State. 2017;42:470–98. https://doi.org/10.1080/10408436.2016.1244642.
Hasue K, Yoshitake K, Matsukawa M. Mixtures of phase-stabilized ammonium nitrate and tetrazoles as new gas-generating agent compositions. Cent Eur J Energ Mater. 2016;13:247–60.
Popławski D, Hoffmann J, Hoffmann K. Effect of carbonate minerals on the thermal stability of fertilisers containing ammonium nitrate. J Therm Anal Calorim. 2016;124:1561–74. https://doi.org/10.1007/s10973-015-5229-1.
Baraza X, Pey A, Giménez J. The self-sustaining decomposition of ammonium nitrate fertiliser: Case study, Escombreras valley. Spain J Hazard Mater. 2020;387:121674. https://doi.org/10.1016/j.jhazmat.2019.121674.
Menicacci E, Rotureau P, Fayet G, Adamo C. Toward the Mechanistic understanding of the additives’ role on ammonium nitrate decomposition: Calcium carbonate and calcium sulfate as case studies. ACS Omega. 2020;5:5034–40. https://doi.org/10.1021/acsomega.9b03964.
Han Z, Sachdeva S, Papadaki MI, Mannan S. Effects of inhibitor and promoter mixtures on ammonium nitrate fertilizer explosion hazards. Thermochim Acta. 2016;624:69–75. https://doi.org/10.1016/j.tca.2015.12.005.
Sun J, Sun Z, Wang Q, Ding H, Wang T, Jiang C. Catalytic effects of inorganic acids on the decomposition of ammonium nitrate. J Hazard Mater. 2005;127:204–10. https://doi.org/10.1016/j.jhazmat.2005.07.028.
Pandey M, Jha S, Kumar R, Mishra S, Jha RR. The pressure effect study on the burning rate of ammonium nitrate-HTPB-based propellant with the influence catalysts. J Therm Anal Calorim. 2012;107:135–40. https://doi.org/10.1007/s10973-011-1718-z.
Yang M, Chen X, Yuan B, Wang Y, Rangwala AS, Cao H, et al. Inhibition effect of ammonium dihydrogen phosphate on the thermal decomposition characteristics and thermal sensitivity of ammonium nitrate. J Anal Appl Pyrolysis. 2018;134:195–201. https://doi.org/10.1016/j.jaap.2018.06.008.
Babrauskas V, Leggett D. Thermal decomposition of ammonium nitrate. Fire Mater. 2020;44:250–68. https://doi.org/10.1002/fam.2797.
Kaljuvee T, Edro E, Kuusik R. Influence of lime-containing additives on the thermal behaviour of ammonium nitrate. J Therm Anal Calorim. 2008;92:215–21. https://doi.org/10.1007/s10973-007-8769-1.
Myka A, Zdunek A, Rusek P. Thermal analysis methods and their application in studies of the effect of additives on the thermal decomposition of ammonium nitrate. Przemysl Chem. 2022;1:46–52.
Han Z, Sachdeva S, Papadaki MI, Mannan MS. Ammonium nitrate thermal decomposition with additives. J Loss Prev Process Ind. 2015;35:307–15. https://doi.org/10.1016/j.jlp.2014.10.011.
Vargeese AA, Muralidharan K, Krishnamurthy VN. Thermal stability of habit modified ammonium nitrate: Insights from isoconversional kinetic analysis. Thermochim Acta. 2011;524:165–9. https://doi.org/10.1016/j.tca.2011.07.009.
Shiota K, Matsunaga H, Miyake A. Effects of amino acids on solid-state phase transition of ammonium nitrate. J Therm Anal Calorim. 2017;127:851–6. https://doi.org/10.1007/s10973-016-5416-8.
Gorbovskiy K, Kazakov A, Norov A, Malyavin A, Mikhaylichenko A. Properties of complex ammonium nitrate-based fertilizers depending on the degree of phosphoric acid ammoniation. Int J Ind Chem. 2017;8:315–27. https://doi.org/10.1007/s40090-017-0121-4.
Kaniewski M, Huculak-Mączka M, Zieliński J, Biegun M, Hoffmann K, Hoffmann J. Crystalline phase transitions and reactivity of ammonium nitrate in systems containing selected carbonate salts. Crystals. 2021;11:1250. https://doi.org/10.3390/cryst11101250.
Artyukhov A, Volk I, Krmela J, Chernenko A, Ospanov D. Production of ammonium nitrate with nanoporous structure: The influence of technological parameters on quality of granules. Int J Adv Manuf Technol. 2022;121:1697–706. https://doi.org/10.1007/s00170-022-09449-w.
Taran AL, Ostanina OI, Taran AV, Bespalova VO. Analysis of the national and foreign quality requirements for basic mineral nitrogenous fertilizers, and technical solutions for improving their quality. Chem Petrol Eng. 2016;52:10–4. https://doi.org/10.1007/s10556-016-0138-0.
Elzaki BI, Zhang YJ. Surface modification of ammonium nitrate by coating with surfactant materials to reduce hygroscopicity. Defence Technology. 2019;15:615–20. https://doi.org/10.1016/j.dt.2019.01.004.
Tyc A, Penkala S, Biegun M, Nieweś D, Huculak-Mączka M, Hoffmann K. The effectiveness of commercial anticaking agents for ammonium nitrate fertilizers. Ecol Chem Eng A. 2020;26:127–35. https://doi.org/10.2428/ecea.2019.26(1-2)11.
Assunção AGL, Cakmak I, Clemens S, González-Guerrero M, Nawrocki A, Thomine S. Micronutrient homeostasis in plants for more sustainable agriculture and healthier human nutrition. J Exp Bot. 2022;73:1789–99. https://doi.org/10.1093/jxb/erac014.
REGULATION (EU) 2019/1009 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. 2019.
Chugh G, Siddique KHM, Solaiman ZM. Iron fortification of food crops through nanofertilisation. Crop Pasture Sci. 2022;73:736–48. https://doi.org/10.1071/CP21436.
Almendros P, Obrador A, Alvarez JM, Gonzalez D. Zn-DTPA-HEDTA-EDTA application: a strategy to improve the yield and plant quality of a barley crop while reducing the N application rate. J Soil Sci Plant Nutr. 2019;19:920–34. https://doi.org/10.1007/s42729-019-00090-3.
Szerement J, Szatanik-Kloc A, Mokrzycki J, Mierzwa-Hersztek M. Agronomic biofortification with Se, Zn, and Fe: An effective strategy to enhance crop nutritional quality and stress defense—a review. J Soil Sci Plant Nutr. 2022;22:1129–59. https://doi.org/10.1007/s42729-021-00719-2.
Rengel Z. Availability of Mn, Zn and Fe in the rhizosphere. J Soil Sci Plant Nutr. 2015;15:397–409. https://doi.org/10.4067/S0718-95162015005000036.
Yu G, Duh YS, Yang X, Li Y, Chen Y, Li Y, et al. Holistic Case Study on the explosion of ammonium nitrate in Tianjin port. Sustainability. 2022;14(6):A3429. https://doi.org/10.3390/su14063429.
Sivaraman S, Varadharajan S. Investigative consequence analysis: A case study research of beirut explosion accident. J Loss Prev Process Ind. 2021;69:104387. https://doi.org/10.1016/j.jlp.2020.104387.
Kaniewski M, Hoffmann K, Hoffmann J. Influence of selected potassium salts on thermal stability of ammonium nitrate. Thermochim Acta. 2019;678:178313. https://doi.org/10.1016/j.tca.2019.178313.
Halstead WD. Thermal decomposition of ammonium sulphate. J App Chem. 1970;20:129–32. https://doi.org/10.1002/jctb.5010200408.
Zelenková G, Slovák V. Decomposition of ammonium salts by quantitative TG-MS. J Therm Anal Calorim. 2022;147:15059–68. https://doi.org/10.1007/s10973-022-11747-0.
Kazakov AI, Ivanova OG, Kurochkina LS, Plishkin NA. Kinetics and mechanism of thermal decomposition of ammonium nitrate and sulfate mixtures. Russ J Appl Chem. 2011;84:1516–23. https://doi.org/10.1134/S1070427211090102.
Cagnina S, Rotureau P, Fayet G, Adamo C. The ammonium nitrate and its mechanism of decomposition in the gas phase: A theoretical study and a DFT benchmark. Phys Chem Chem Phys. 2013;15:10849–58. https://doi.org/10.1039/C3CP50368B.
Cheng L, Li W, Li Y, Yang Y, Li Y, Cheng Y, et al. Thermal analysis and decomposition kinetics of the dehydration of copper sulfate pentahydrate. J Therm Anal Calorim. 2019;135:2697–703. https://doi.org/10.1007/s10973-018-7595-y.
Klimova I, Kaljuvee T, Türn L, Bender V, Trikkel A, Kuusik R. Interactions of ammonium nitrate with different additives: Thermodynamic analysis. J Therm Anal Calorim. 2011;105:13–26.
Coombs PG, Munir ZA. The decomposition of iron(III) sulfate in air. J Therm Anal. 1989;35:967–76. https://doi.org/10.1007/BF02057253.
Warner TE, Bancells MM, Lund PB, Lund FW, Ravnsbæk DB. On the thermal stability of manganese(II) sulfate and its reaction with zeolite A to form the sodalite Na6Mn2[Al6Si6O24](SO4)2. J Solid State Chem. 2019;277:434–40. https://doi.org/10.1016/j.jssc.2019.06.038.
Rego ASC, Rio R, Navarro CS, Brocchi EA, Souza RFM. Kinetic modeling of the thermal decomposition of zinc sulfate through a global optimization method. Metall Mater Trans B. 2022;53:4105–13. https://doi.org/10.1007/s11663-022-02670-8.
Acknowledgements
We would like to thank the organizers of the 13th ESTAC conference (Palermo, 19 – 22 Sept) during which it was made possible to present partial results contained in this paper.
This research was funded by the Ministry of Science and Higher Education of Poland within a frame of science subsidy for 2022 which was realized in the Department of Engineering and Technology of Chemical Processes, Wroclaw University of Science and Technology (no. 8211104160 — K24W03D05).
Funding
Ministerstwo Edukacji i Nauki, 8211104160-K24W03D05
Author information
Authors and Affiliations
Contributions
MK contributed to conceptualization; MB and MK helped in methodology, formal analysis and investigation and writing — review and editing; MB contributed to writing — original draft preparation; JH helped in funding acquisition and resources; JH, MK and all authors have supervised, read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaniewski, M., Biegun, M. & Hoffmann, J. Thermal stability of systems containing ammonium nitrate and sulfate salts: an experimental study. J Therm Anal Calorim 148, 13051–13064 (2023). https://doi.org/10.1007/s10973-023-12328-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12328-5