Abstract
The mechanisms of the enzymatic polymerization of flavonoids, including quercetin, rutin and catechins, have been fairly well researched, but the properties of polymeric forms of flavonoids still require in-depth analysis. The products of enzymatic polymerization are oligomeric flavonoids. The antioxidant and antimicrobial properties of many oligomeric flavonoids have been described in the literature. However, data on the thermal properties of oligomeric flavonoids are lacking and the supplementing of these deficiencies is a scientific novelty of this work. The aim of this study is to determine the effect of enzymatic polymerization on thermal stability of oligomeric flavonoids. As part of the work, oligomeric quercetin, rutin and catechin were prepared by the enzymatic polymerization reaction with laccase and horseradish peroxidase enzymes. The oligomeric structure of the flavonoids was confirmed by spectroscopic (Fourier transform infrared spectroscopy (FTIR) and UV–visible spectroscopy (UV–Vis)) as well as chromatographic (ultra-performance liquid chromatography (UPLC) and gel permeation chromatography (GPC)) methods. Thermal properties of oligomeric flavonoids were investigated using differential scanning calorimetry (DSC) and thermogravimetry (TG). Moreover, the antioxidant and antimicrobial properties of oligomeric forms of selected polyphenols were investigated. Based on DSC analysis, it was found that the common feature of all oligomeric flavonoids was a higher final oxidation temperature and a higher oxidation enthalpy than the reference flavonoids. Thermogravimetric analysis showed that oligomeric poly(flavonoids)-laccase had better thermal stability, which correlated with higher molar mass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyphenols are secondary metabolites commonly found in the plant kingdom. Phenols have been classified into two main groups: flavonoids (subgroups: flavanols, flavanones, flavonols, flavonones, anthocyanins and isoflavones) and non-flavonoids (such as phenolic acids, xanthones, stilbenes and lignans). Phenolic compounds have an aromatic ring with at least one hydroxyl group, and their structure can range from a simple molecule to a complex polymer with high molecular mass [1]. Polyphenolic compounds are of great interest due to their antioxidant properties and the possibility of being used as natural preservatives in the food industry. Moreover, these phytochemicals are valued for their pro-health benefits and potential application for therapeutic purposes. A relationship between polyphenol consumption and human health has been established, particularly with respect to cardiovascular disease, hypertension, obesity and cancer [2].
Flavonoids are a class of compounds most characteristic of higher plants. They are biocompounds analysed by many scientists and valued for their interesting properties. Flavonoids are an important ingredient in various pharmaceutical, medicinal and cosmetic applications, which is related to their antioxidant, anti-inflammatory, anti-mutagenic and anti-carcinogenic properties [3, 4]. The activity of flavonoids is closely related to the chemical structure of these compounds. In numerous publications, attempts have been made to determine the structure–activity relationships (SAR) for the antioxidant and pharmacological properties of flavonoids [5,6,7,8,9]. One of the structural elements influencing the activity of flavonoids is the degree of polymerization. However, the relationship between the degree of polymerization and the properties of these phytochemicals is not well understood [6]. Several literature reports describe the SAR of natural tannins, i.e. polymeric forms of catechins. Due to the variety and complexity of tannins, the SAR are difficult to establish. The studies indicated that, to some extent, an increasing degree of polymerization increased the effectiveness of the procyanidins against various radicals. The extensive conjugation between 3-OH and B ring catechol groups, together with abundant β4→8 linkages, gave the polymeric form significant radical scavenging properties by increasing the stability of its radical [10, 11]. Procyanidin dimers and trimers were more effective than monomeric flavonoids against superoxide anions; however, the dimer and trimer activities were similar [11]. Tetramers showed greater activity against peroxide-mediated oxidation than trimers, while heptamers and hexamers had significantly better superoxide scavenging properties than trimers and tetramers [11].
The activity of flavonoids is closely related to the chemical structure of these compounds, as well as to their physical state. The amorphous compounds have much higher solubility than the crystalline, and thus, flavonoids have higher activity as drugs [12,13,14] Kanaze and co-authors showed that the solubility of both flavanone glycosides and their aglycones was directly affected by the new physical state of the solid dispersions. Due to the amorphous state of the drug or the nanodispersions in the polyvinylpyrrolidone (PVP) matrices, the solubility was enhanced and the authors found it to be 100% at pH 6.8 in a nanodispersion containing 20% by mass of aglycones. Moreover, the improvement in solubility was observed in polyethylene glycol (PEG) and mannitol solid dispersions; however, it was less than in the case of PVP nanodispersions due to the presence of medicinal compounds in the crystalline state in both matrices [12].
The thermal stability of polyphenols, including flavonoids, may be an important parameter during their processing in packaging for the food and pharmaceutical industries. The pharmaceutical and food industries use various types of polymeric packaging, consisting of a polymer matrix and a number of additives, including stabilizers. Processing additives, such as stabilizers for this type of packaging must meet many requirements, including limited migration into the packed product. Polyphenols and their polymeric forms seem to be a potential stabilizer of packaging materials [15]. Flavonoids have beneficial antioxidant properties but cannot be degraded during plastics processing. Therefore, it is important to know their thermal stability, which is required during the production of polymeric packaging materials with stabilizers. Knowledge of the thermal stability of flavonoids and the changes in their antioxidant activity during heat treatment seems to be important both in the field of food processing [16], as well as in the processing of packaging materials in this industry. Few scientific publications have been devoted to the analysis of the influence of the structure of monomeric flavonoids [16,17,18] and their polymeric forms on their thermal stability, both those occurring in nature and those synthesized in chemical reactions [19,20,21,22,23,24]. Chaaban et al. [16] investigated the relationship between thermostability, flavonoid structure and antioxidant activity of six flavonoids (rutin, naringin, eriodictyol, mesquitol, luteolin and luteolin 7-O glucoside). Temperature had a significant influence on the stability of the flavonoids as well as their biological activity. Due to their structure, flavonoids can be more or less sensitive to heat treatment. Glycosylated flavonoids showed better resistance to heat treatment compared to aglycone flavonoids. Thermal degradation depended on structural solidity of the phytochemicals. The double bond required more energy to degrade [16]. In the case of polymeric flavonoids synthesized as a result of chemical reactions, only a few publications have examined the thermal stability of the compounds obtained [19,20,21,22,23,24]. The relationship between polymeric flavonoids and their thermal properties has been poorly studied. Polymeric forms of flavonoids have been obtained as a result of several different chemical reactions, such as enzymatic polymerization [20, 23, 25,26,27,28,29,30,31,32,33], as well as polymerization with a cross-linking compound [19, 21, 22, 24]. For poly(flavonoids) synthesized by reactions with a cross-linking agent, it was found that the polymeric forms of rutin [21] and quercetin [22], as well as catechin [19] and naringenin [24], had better thermal stability. Only one publication related to enzymatic polymerization [20] has described the thermal stability of polymeric quercetin. Poly(quercetin) obtained from the reaction with the horseradish peroxidase (HRP) enzyme was thermally stable due to the formation of new catechol-catechol bonds in the polymeric backbone [20].
Although enzymatic polymerization is a well-described method of obtaining polymeric forms of flavonoids, and the mechanisms of these reactions are known [20, 23, 25,26,27,28,29,30,31,32,33], data on the influence of polymerization on the thermal behaviour of polymeric forms of flavonoids and the relationship of the polymeric structure with these properties are lacking. The authors of these scientific publications focused mainly on characterizing the structure of the polymerization product and determining the antioxidant and antimicrobial properties, as well as finding the SAR for poly(flavonoids) synthesized in enzymatic reactions. Poly(flavonoids) were produced using two types of enzyme, laccase and peroxidase. The laccase enzyme was used to synthesize polymeric forms of catechin [33], rutin [28, 29], quercetin [27] and esculin [30]. As a result of the reaction with horseradish peroxidase, polymer structures of catechin [26, 31, 32] and quercetin [20] were obtained. The biological activity of the polymeric flavonoids differed from that of the reference monomer compounds. Chebil et al. [30] showed that the antioxidant activity of poly(flavonoids) may depend on the molecular mass of the biocompounds and the type and location of bonds (Mw, PDI, C–C or C–O bridges). The authors found a decrease in the anti-radical activity of poly(rutin) with an increase in its molecular mass Mw [30]. On the other hand, other research methods showed a different dependence of the antioxidant capacity in the polymeric flavonoids. The poly(catechin) obtained in the enzymatic reaction was characterized by a stronger inhibitory effect on DPPH free radicals [32, 33]. In the case of the xanthine oxidase inhibition test, investigation found that enzymatic polymerization of polyphenols (rutin, esculin, catechin and epigallocatechin gallate) increases their antioxidant capacity [28,29,30].
The aim of this study is to analyse the thermal properties of polymeric flavonoids obtained by enzymatic polymerization with the laccase and horseradish peroxidase enzymes. Flavonoids differing in chemical structure and belonging to different subgroups were selected for the study: quercetin (flavonol) and its glycoside rutin, as well as catechin (flavan-3-ol). The oligomeric structure of the flavonoids was confirmed by spectrophotometric and chromatographic methods. The influence of enzymatic polymerization, including the type of enzyme used, on the thermal stability of the macromolecular compounds synthesized was analysed. The antioxidant and antimicrobial properties were also investigated and were correlated with the thermal behaviour of poly(flavonoids). The analysis of the thermal properties of oligomeric flavonoids, establishing the correlation between the thermal stability and the antioxidant and antimicrobial activity of poly(flavonoids), is a scientific novelty and is an extension of the current research on polymeric forms of flavonoids obtained in enzymatic polymerization reactions.
Experimental
Materials and methods
Preparation of polymeric forms of flavonoids by reaction with enzymes
Quercetin (≥ 95% HPLC, MW: 302.24 g mol–1, Sigma-Aldrich), rutin (≥ 94% (HPLC), MW: 610.52 g mol–1, Sigma-Aldrich) and catechin (( +)-catechin ≥ 98% HPLC, MW: 290.27 g mol–1, Sigma-Aldrich) were polymerized by reaction with laccase (enzyme from Aspergillus sp., Sigma-Aldrich) and horseradish peroxidase (HRP) (type VI-A, Sigma-Aldrich). The glycoprotein laccase EC 1.10.3.2 is an extracellular multicopper enzyme. It can oxidize aromatic and nonaromatic compounds [34]. HRP belongs to the ferroprotoporphyrin peroxidase group and is an enzyme produced from horseradish roots (Amoracia rusticana). It is a single chain polypeptide containing four disulfide bridges. The enzyme readily combines with hydrogen peroxide, and the resultant HRP-H2O2 complex can oxidize a wide variety of hydrogen donors [35].
The synthesis of polymeric flavonoids with laccase was performed according to a published method [29] but with changes. Flavonoids (0.2 g) were placed in vials, and 6 mL of methanol (pure p.a., POCH) and 14 mL of phosphate buffer pH 7.4 (1 M, Sigma-Aldrich) were added. The materials were mixed at room temperature using a magnetic stirrer until the flavonoids were completely dissolved. Then, 1000 units of laccase enzyme were added to the flavonoid solutions. In the next step, the solutions were gently mixed with a magnetic stirrer for 48 h at room temperature and open to air. The solutions were dialyzed in distilled water using 8kD dialysis membranes. After dialysis, the solutions were lyophilized to obtain polymeric forms of flavonoids.
The polymerization of quercetin, rutin and catechin with HRP was carried out at atmospheric pressure and at room temperature, according to the methodology of Bruno et al. [20] but with changes. 6 mL of phosphate buffer (pH 7, 0.1 M, CHEMPUR) and 4 mL of ethanol (96%, pure p.a., POCH) were mixed, and then 10 mg of flavonoid was added to the solution. When the flavonoid was completely dissolved, 4 mg of HRP enzyme was added. The polymerization was activated by adding 0.03% hydrogen peroxide (1.5 mL, pure, CHEMPUR) to the solution. The solutions of all flavonoids were mixed with a magnetic stirrer under the following conditions: time 20 h, air access, room temperature. Similar to the polymerization with laccase, the solutions were dialyzed and lyophilized.
Analysis of the polymeric structure of flavonoids
Scanning electron microscopy (SEM)
The morphology of powders of the flavonoids and their polymeric forms was evaluated on the basis of photos obtained with a scanning electron microscope (SEM) (LEO 1530 (Carl Zeiss AG). The photos were done at magnifications of: 1000, 10,000 and 50,000×.
FTIR and UV–visible spectroscopy (UV–Vis) spectroscopy
Fourier transform infrared (FTIR) spectroscopy (Nicolet 670 FTIR spectrophotometer, Thermo Fisher Scientific) was utilized to determine specific chemical groups of flavonoids and poly(flavonoids). The oscillation spectra were recorded in the wave number range 4000–400 cm−1. The ATR accessory equipped with a single-reflection ATR diamond crystal was applied for the tests. Measurements with a UV–Vis spectrophotometer (Evolution 220, Thermo Fisher Scientific) were made for samples dissolved in ethanol (flavonoids) and in distilled water (polymeric flavonoids) at a concentration of 1 mg m-1. UV–Vis spectra of the biocompounds at wavelengths of 190–1100 nm were recorded.
Gel permeation chromatography (GPC) and UPLC-PDA chromatography
To analyse the molecular masses and molecular mass distributions of polymeric forms of flavonoids, gel permeation chromatography (GPC) was used. GPC analysis was made using a Wyatt instrument equipped with two Perfect Separation Solutions columns and one guard column [linear GRAM (10 µm, Mn between 800 Da and 1,000,000 Da)], light scattering and differential refractometric detectors (Wyatt). Measurements were taken in DMF with 50 mmol LiBr as eluent at a flow rate of 1 mL min−1. Polystyrene (PS) standards (PS from Mp = 1,306 Da to Mp = 1,210,000 Da) were utilized for calibration. Chromatographic separations of the flavonoid and polymerized flavonoid samples were performed using the UPLC-PDA liquid chromatography technique. The study was carried out using a Waters Acquity H-class liquid chromatograph equipped with a UV detector with a diode matrix. Samples of flavonoids and poly(flavonoids) were dissolved in methanol and filtered through a 0.45-µm GHP syringe filter. Prior to analysis, the samples were diluted with methanol to a concentration of 0.1 mg/mL. Analysis conditions were: column: acquity UPLC BEH C18, 1.7 μm; 2.1 × 100 mm; column temperature: 30 °C; detection at λ = 290 nm; injection volume: 5 μL; mobile phase A: water + 0.5% formic acid; mobile phase B: acetonitrile + 0.5% formic acid; mobile phase flow: 0.3 mL/min; gradient program: 0 min—A-98%, B-2%; 3.0 min—A-98%, B-2%; 10.0 min—A-85%; B-15%, 15.0 min—A-70%, B-30%; 20.0 min—A-10%, B-90%; 25.0 min—A-98%, B-2%; 26.0 min—A-98%, B-2%; total program time: 26 min.
Thermal properties analysis
The thermal stability of quercetin, rutin, catechin and its polymeric forms was determined by thermogravimetric analysis (TG). The powders (approximately 10 mg) were placed in alumina crucibles and heated from 25 to 800 °C under an argon atmosphere (flow 50 mL min–1) at a rate of 5 °C min–1 using a Mettler Toledo Thermobalance (TA Instruments). Differential scanning calorimetry (DSC) (Mettler Toledo DSC analyser, TA 2920, TA Instruments) was used to analyse the temperature ranges of flavonoid and poly(flavonoid) phase changes. Approximately 5 mg of the powders was placed in aluminium pans. The measurements were performed under the following conditions: temperature range −80 to 400 °C, heating rate 10 °C min–1, air flow 60 mL min–1.
Analysis of antioxidant and antimicrobial properties
Antioxidant activity
The ability of biocompounds to reduce free radicals was determined by analogous methods using ABTS+⋅ (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid) cation radicals and DPPH⋅ (1,1-diphenyl-2-picrylhydrazyl) radicals. The degree of reduction of ABTS+⋅ or DPPH⋅ radicals was calculated using the Eq. (1):
where A0 is the absorbance of the reference sample without biocompound and A1 is the absorbance of the sample with flavonoid or polymeric form of flavonoid.
As part of the activity tests, the ability of phytochemicals to reduce transition metal ions (iron and copper) was also determined using FRAP (ferric reducing antioxidant power; Fe3+ → Fe2+) and CUPRAC (cupric reducing antioxidant capacity; Cu2+ → Cu1+) methods. The quantitative ability of flavonoids and poly(flavonoids) to reduce transition metal ions was computed by comparing the change in absorption (ΔA) of the sample tested with the value of ΔA determined for reference solutions without phytochemicals. The ΔA value obtained was directly proportional to the concentration of the antioxidant substance.
For ABTS, DPPH, FRAP and CUPRAC tests, solutions of quercetin, rutin, poly(quercetin) and poly(rutin) at a concentration of 1.0 mg mL–1, as well as catechin and poly(catechin) at a concentration of 0.1 mg mL–1 were prepared. Catechin solutions were prepared at a lower concentration than other samples because their activity at higher concentration exceeded the measuring range of the methods. The detailed methodology of ABTS, DPPH, FRAP and CUPRAC determinations was described by the authors in previous publications [36, 37].
Antimicrobial properties
The antimicrobial activity was determined by the well diffusion method. The media of TSA (bacteria) and MEA (yeasts and moulds) were seeded with suspensions of the appropriate microorganisms in physiological saline, and then the wells were cut with a sterile 15 mm diameter corkborer. 200 µL of suspensions of flavonoids and poly(flavonoids) prepared in DMSO at concentrations of 1, 5 and 10 mg mL–1 was introduced into the wells. After 48 h of incubation at 30 °C, the growth inhibition zones around the wells were analysed. The following microorganisms were used in the research: bacteria—Escherichia coli (Collection no. ATCC 10536), Staphylococcus aureus (Collection no. ATCC 6538), Bacillus subtilis (Collection no. NCAIM 01644); fungus–yeast Candida albicans (Collection no. ATCC 10231) and moulds Aspergillus niger (Collection no. ATCC 16404), where ATCC is American Type Culture Collection; NACIM is National Collection of Agricultural and Industrial Microorganisms.
Results and discussion
Confirmation of the polymeric form of flavonoids
According to the literature, oligomeric forms of flavonoids are obtained as the product of enzymatic polymerization [20, 23, 28, 31].
The mechanism of quercetin polymerization with HRP has been explained [20]. The polymerization with HRP was a reaction involving the initial two-electron oxidation of the ferric enzyme to produce an oxidized intermediate (HRP-I) by adding hydrogen peroxide. In the next step, the monomeric form of quercetin was oxidized by the intermediate (HRP-I) to form the monomeric radical. Radical species joined together to form dimers first, then trimers, which was repeated until a substantial oligomer size was obtained.
The quercetin glycoside is rutin. Rutin oligomers are identified as di-, tri-, tetra-, penta- and hexamers. Regardless of the reaction conditions (pH, temperature, enzyme concentration, etc.), no higher molecular masses were observed, while the relative percentage of each oligomer was dependent on the experimental conditions. The research results suggested a symmetrical structure of the oligomeric rutin that resulted from the C2′–C2′ bridge between the two rutin units, which meant that both C–C and C–O bonds were formed during the enzymatic oligomerization of rutin [28].
Hosny et al. described the mechanism of enzymatic polymerization of catechin [31]. Three different C–C biphenyl dimers were formed by reaction with HRP. The enzyme catalysed the H2O2-dependent oxidative coupling of ( +)-catechin. According to the research, the enzyme laccase catalysed the formation of two new adducts of catechin and hydroquinone. Most often, ( +)-catechin polymers were formed by couplings between ring A of one unit and ring B of the other unit in a head-to-tail polymerization reaction. Using peroxidase dependent on H2O2, as well as chemical oxidation of ( +)-catechin under alkaline conditions, 8-hydroxy-( +)-catechin and catechin dimers (dehydrodicatechins) were obtained. The chemical structures of dehydrodicatechins differed from one another in the type of the biphenyl or phenyl ether (C–C or C–O–C) types of linkage, as well as the position of the interflavan linkage and relative conformations of biphenyl or biphenyl ether moieties [31].
Figure 1 shows exemplary schemes of enzymatic polymerization of quercetin, rutin and catechin along with suggested structures of oligomers (elaboration based on the previously cited literature data—quercetin [20], rutin [28] and catechin [31].
The mechanism of enzymatic polymerization of flavonoids, as well as the structure of the products obtained, has been quite well described in the literature [20, 23, 28, 31]. Therefore, as an introduction to the research, only the polymeric forms of quercetin, rutin and catechin used for further analyses were confirmed.
The enzymatic polymerization of flavonoids was accompanied by a clear change in the colour of quercetin, rutin and catechin, as well as a change in the morphology of the biochemicals. Figure 2 shows photographs from a camera and from an SEM of flavonoids and their polymeric forms. During polymerization, all test compounds changed colour from yellow (quercetin, rutin) or orange (catechin) to dark brown. The SEM images showed visible changes in the morphology of the samples. Monomeric flavonoids had a needle structure. Rutin was characterized by the smallest size of “needles” in its structure, but this needle-like morphology was visible in the photos at higher magnifications (50,000× and more). The poly(flavonoids) obtained as a result of the polymerization reaction with laccase and HRP showed a lamellar, slightly spongy structure. The needle structure in the polymerization products was invisible.
The FTIR (Fig. 3 (A.1., B.1., C.1.)) and UV–Vis spectra (Fig. 3 (A.2., B.2., C.2.)) identified characteristic functional groups in the polymeric flavonoids. The bands specific to poly(flavonoids) were in the range 3000–3500 cm−1, suggesting the presence of the hydrogen bond of the OH group for phenol, and also 1550–1650 cm−1 corresponding to the o-quinone [20, 38]. Other significant bands were identified in the following ranges: 1300–1200 cm−1—the characteristic aryl stretching vibrations, and a band with a peak at approx. 1060 cm−1 corresponding to C–CO–C groups in ketones [21,22,23]. It seems interesting that the FTIR spectra of poly(flavonoids) synthesized by reactions with various enzymes were identical for pairs poly(quercetin)-laccase and poly(quercetin)-HRP, poly(rutin)-laccase and poly(rutin)-HRP, and poly(catechin)-laccase and poly(catechin)-HRP. As a result of enzymatic polymerizations with the use of different types of enzyme, it is possible to obtain repeat products with identical structure.
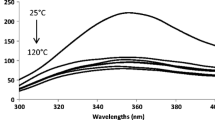
Another spectrophotometric method, UV–Vis spectra (Fig. 3 (A.2., B.2., C.2.)), also confirmed the polymeric structure of flavonoids. Monomeric quercetin, rutin and catechin had two specific peaks at 284 and 291 nm. These flavonoid peaks were also identified by other authors. For example, the UV–Vis spectrum of rutin showed two absorption maxima at 282 and 359 nm, corresponding to the π-π* transition of aromatic electrons [28]. The poly(flavonoids) peaks were wide, shifted and were identified in the range 280–400 nm. Moreover, the peaks had a specific tail which, in the range 390–600 nm, corresponded to conjugation created by the polymerization [20]. According to the literature, the 359 nm band of the oligomeric rutin is wide and shifted hypsochromically by about 11 nm, which suggested the implication of the rutin B ring in the formation of oligomeric forms [28].
Chromatographic methods such as ultra-performance liquid chromatography (UPLC) and GPC were also used to confirm the enzymatic polymerization of flavonoids. The results are exemplified by polymerization with the enzyme laccase. In Fig. 4(A.1), (A.2), (A.3), UPLC chromatograms are summarized, and in Fig. 4(B.1) gel permeation chromatography GPC traces of poly(flavonoids) are shown. In Fig. 4(B.2), the characteristics of flavonoids and poly(flavonoids)-laccase (where the Mn [M] and Mw/Mn were determined by GPC (PS standard)) are compiled.
Chromatographic separations of the flavonoid and polymeric flavonoid samples were performed using the UPLC-PDA liquid chromatography technique. Due to the lack of standards of polymeric flavonoid forms, it was possible to compare the chromatograms of samples before and after polymerization without qualitative analysis. All comparisons were made at 290 nm. A clear tendency was found for samples after polymerization with laccase for disappearance of the flavonoid UPLC chromatographic peaks. A comparison of the quercetin and poly(quercetin)-laccase chromatographs (Fig. 4(A.1)) showed that the quercetin chromatographic peak at a retention time of 16.6 min had gone after the enzymatic reaction. In Fig. 4(A.2), the rutin peak disappeared at a retention time of 13.3 min, and in Fig. 4(A.3) disappearance of the catechin peak at a retention time of 8.9 min was observed. The disappearance of the chromatographic peaks of monomeric flavonoids confirmed the structural changes of compounds that occurred as a result of polymerization with laccase.
In Fig. 4(B.2), characteristics of flavonoids and poly(flavonoids)-laccase and poly(flavonoids)-HRP by GPC are summarized. The flavonoids quercetin, rutin and catechin are low molecular mass compounds, and therefore, the monomeric form samples were below the measuring range of GPC chromatography. The GPC results showed and confirmed that the poly(flavonoids) obtained by the reaction with laccase were oligomers, as suggested by the literature data [20, 23, 28, 31]. Poly(rutin)-HRP was also an oligomer. In contrast, poly(quercetin)-HRP and poly(catechin)-HRP were below the quantification limit of the GPC chromatograph. The FTIR and UV–Vis analyses showed that polymerization with laccase and HRP resulted in polymeric forms of all flavonoids, and the spectrophotometric spectra were repeated and analogous to the two polymerization methods. The results of the FTIR, UV–Vis and GPC analyses suggest that poly(quercetin)-HRP and (poly)catechin)-HRP were oligomeric structures with very low molecular mass. The polydispersity index PDI (Mw/Mn) is used to describe the molar mass heterogeneity of a polymer. The PDI value of monodisperse (homogeneous) polymers is 1. Biopolymers are an example of such polymers [39]. The PDI for poly(quercetin)-laccase and poly(rutin)-laccase was approximately 1.17, and for poly(catechin)-laccase it was 1.106. The samples showed some heterogeneity in the molar mass, which could be caused by the presence of oligomers with different chain lengths, as well as by the polymerization conditions. The poly(rutin)-HRP sample exhibited the highest PDI, which meant that this oligomer was characterized by the greatest heterogeneity of molar mass. Molar mass heterogeneity can affect the thermal properties of the samples, as described later in the manuscript.
Summarizing, in accordance with the assumptions of the literature, oligomeric structures were obtained as a result of the polymerization of flavonoids with laccase and HRP enzymes. Specific functional groups for polymeric forms were present on the FTIR and UV–Vis spectra of poly(flavonoids)-laccase and poly(flavonoids)-HRP, and the spectra were repeatable. On the basis of GPC chromatographic analysis, it was shown that the oligomeric flavonoids obtained in the polymerization reaction with HRP had very low molar masses (below the quantification limit of the GPC apparatus), lower than the molar masses of poly(flavonoids) synthesized in an analogous reaction with laccase.
Thermal properties analysis
Phase transitions by differential scanning calorimetry (DSC) of oligomeric quercetin, rutin and catechin obtained in enzymatic polymerization reactions have not been analysed in the literature so far. The influence of the enzyme used for the polymerization reaction on thermal transformation of the polymeric forms of flavonoids has also not been described in scientific literature. Table 1 and Fig. 5 show the phase transitions of the flavonoid and poly(flavonoids) samples determined by differential scanning calorimetry.
DSC measurements were performed in air in the temperature range − 80 to 400 °C. The DSC curves showed endothermic melting peaks and exothermic oxidation peaks. Oxidation of monomeric flavonoids has been associated with thermo-oxidative degradation. The melting of quercetin and its glycoside rutin was a one-step process. The melting temperature of quercetin was 111.1 °C and of rutin 160.4 °C. However, catechin melting was a two-stage process (two endothermic peaks, 134.3 °C, 171.4 °C). The melting of all polymeric forms of flavonoids began at lower temperatures than the melting of the reference monomers (lower by value: poly(quercetin)-laccase 48.2 °C, poly(quercetin)-HRP 82.7 °C, poly(rutin)-laccase 85.6 °C, poly(rutin)-HRP 131.5 °C, poly(catechin)-laccase 55.1 °C, poly(catechin)-HRP 104.5 °C). This could be due to the presence of short unstable oligomer chains or polymerization residue. Oligomeric forms synthesized by reaction with laccase were characterized by a melting temperature of 62–79 °C. The melting temperatures of poly(flavonoids)-HRP were significantly lower, oscillating in the range of 28–29 °C. The results obtained corresponded well with the analysis of molar masses by GPC. The oligomeric flavonoids obtained by the HRP reaction had very low molecular mass (below GPC quantification), so their melting point could also be lower than poly(flavonoids)-laccase with longer polymer chain lengths. The total enthalpy of melting of the polymeric flavonoids (except for the poly(quercetin)-HRP sample) was greater than that of the reference polyphenols.
The initial oxidation temperature (onset) of the oligomeric forms of quercetin and catechin with laccase and HRP was lower than that of the reference flavonoids, which could be due to the presence of low molecular mass polymer fractions or unreacted polymerization residue. On the other hand, the final (endset) oxidation temperatures were much higher. Poly(quercetin)-laccase had a higher final oxidation temperature To (higher by 40.8 °C) and a higher enthalpy of oxidation ΔHo (about 8 times). The final oxidation temperature of poly(quercetin)-HRP was higher by 56.4 °C and the enthalpy of oxidation ΔHo about 14 times greater. Similar observations were made with polymeric catechin samples. The final oxidation temperature of poly(catechin)-laccase was 61.9 °C higher and poly(catechin)-HRP was 77.1 °C higher than the monomer. Oligomeric catechins were also characterized by a higher enthalpy of oxidation ΔHo: poly(catechin)-laccase about 57 times and poly(catechin)-HRP about 8200 times. Although the oligomeric forms of quercetin and catechin synthesized by reaction with HRP had lower melting points than the monomers, they had significantly higher final oxidation temperatures and enthalpies of oxidation, also greater than the final To and ΔHo of the samples with laccase. Interesting results have been found for the polymeric forms of rutin. Oligomers obtained in different reactions with laccase or HRP were characterized by similar enthalpies and oxidation temperatures. Poly(rutin)-laccase and poly(rutin)-HRP may have had a similar degree of cross-linking of the samples, as well as a similar length of the oligomeric chains. However, due to the greater heterogeneity of the molar mass (greater DPI), the poly(rutin)-HRP sample had a lower melting point than poly(rutin)-laccase. The presence of low molecular mass fractions in the sample may lower the melting point.
A common feature of all the oligomeric flavonoid samples was a higher final oxidation temperature and a higher enthalpy of oxidation than the reference polyphenols. These improved parameters meant a greater resistance to oxidation of poly(flavonoids)-laccase and poly(flavonoids)-HRP. The better oxidation resistance of the oligomeric flavonoids could have been due to the presence of longer oligomeric chains and the cross-linked structure of poly(flavonoids). Moreover, the presence of new catechol-catechol bonds in the polymer backbone and a greater number of active hydroxyl groups resulted in the formation of poly(flavonoids) having better resistance to thermo-oxidation. These factors improved their thermal properties by limiting the access of heat and oxygen to the polymeric flavonoid particles, as well as slowing their thermal decomposition and oxidation reactions.
In a previous publication [19], the authors presented the phase transitions of oligomeric naringenin obtained in the polymerization reaction with laccase and HRP. Similar to this manuscript, polymeric forms of naringenin were more resistant to oxidation. Oligomeric naringenin had higher initial and final oxidation temperatures: poly(naringenin)-laccase—initial To by 13.7 °C and final by 28.2 °C; poly(naringenin)-HRP—initial To by 18.4 °C and final by 23.6 °C, as well as a higher enthalpy of oxidation: ΔHo, poly(naringenin)-laccase about 2 times; poly(naringenin)-HRP about 7.7 times. The poly(naringenin)-laccase and poly(naringenin)-HRP had a lower melting point than the reference flavonoid. It has been reported that this could be due to the presence of low molecular mass polymer fractions [23].
The test results for thermal stability of flavonoids and poly(flavonoids) determined using thermogravimetry are shown in Table 2 and Fig. 6.
Table 2 summarizes the temperatures at which there was a mass loss of the samples, corresponding to 10% (T10), 20% (T20), 30% (T30), 40% (T40), 45% (T45), 50% (T50), 55% (T55), 60% (T60) and 65% (T65). Figure 6 shows the thermogravimetric curves of flavonoids and polymerized forms of quercetin, rutin and catechin.
A few reports in the literature indicate that poly(quercetin) obtained as a result of the reaction with the HRP enzyme was more thermally stable than the monomer due to the formation of new catechol-catechol bonds in the polymer main chain [20].
The thermal decomposition of quercetin (up to 800 °C in inert gas) took place in two stages. The first stage occurred around 120 °C, with a mass loss of 7%. The second stage of quercetin decomposition took place in the temperature range 350–380 °C, and the mass loss was 54.6%. Poly(quercetin)-laccase decomposed in two steps. The first stage of decomposition was recorded around 80 °C, mass loss 5.5%. The second stage of decomposition occurred in the temperature range 150–650 °C. The second stage was accompanied by a mass loss of the sample amounting to 24.9%. Thermal decomposition of poly(quercetin)-HRP was also two-stage—first stage around 65 °C, mass loss 10.13%; second stage 150–650 °C, mass loss 47.58%. Table 2 shows the values of the temperatures at which the mass loss of the poly(quercetin)-laccase and poly(quercetin)-HRP samples occurred. The decomposition of poly(quercetin)-laccase started at a lower temperature than that of quercetin (T10 poly(quercetin)-laccase = 181.6 °C, T10 quercetin = 309 °C). The T50 half-decomposition temperature of poly(quercetin)-laccase was 27.8 °C higher compared to the T50 of monomeric quercetin, and the T55 of poly(quercetin) laccase was higher by 128.3 °C, which indicated higher thermal stability of the polymeric form of quercetin obtained in the reaction with laccase. Thermal decomposition of poly(quercetin)-HRP also started at a lower temperature than the decomposition of monomer (T10 poly(quercetin)-HRP = 122.5 °C) Decomposition temperature of T55 poly(quercetin)-HRP was 22.5 °C higher compared to T55 of the reference flavonoid, while the T60 of poly(quercetin)-HRP was higher by as much as 77 °C, which suggested higher thermal stability of the polymeric quercetin synthesized in the reaction with the HRP enzyme. Poly(quercetin)-laccase showed slightly better thermal stability than poly(quercetin)-HRP.
Poly(rutin)-laccase and poly(rutin)-HRP showed very similar paths of the thermal degradation for all samples analysed. Like the thermal degradation of quercetin, the degradation of rutin also had two steps. The first stage was recorded at 170 °C (mass loss 7.5%), and the second in the temperature range 250–320 °C (mass loss 58.8%). Oligomeric forms of rutin decomposed in stages. The first stage of poly(rutin)-laccase decomposition occurred around 160 °C (mass loss 5.9%). The second stage of decomposition was found in the temperature range 207–320 °C (mass loss of the sample 35.5%). Poly(rutin)-HRP thermally decomposed in two stages: first stage at around 65 °C, mass loss 8.76%; the second stage at 150–640 °C, mass loss of the sample 40.26%. The degradation of poly(rutin)-laccase (Table 2) started at a similar temperature as that of the reference rutin (T10 poly(rutin)-laccase = 247.5 °C, T10 rutin = 247 °C). However, the temperature of decomposition T10 of poly(rutin)-HRP was found at a lower temperature (T10 = 209 °C). The final decomposition temperatures T60 and T65 of the polymeric rutin-laccase were 6.6 °C higher than the T60 of rutin and 96 °C higher than the T65 noted for rutin. The T60 of poly(rutin)-HRP was equal to 658 °C and was 68 °C higher than the T60 of the reference rutin, indicating higher thermal stability of the polymeric form of rutin with HRP.
Monomeric catechin, similarly to quercetin and rutin, also thermally decomposed in two stages: first stage temperature 200 °C, mass loss 4.9%; the second stage temperature range 282–327 °C, mass loss 52%. Oligomeric catechins obtained in the polymerization reactions with the laccase and HRP enzymes decomposed in two stages. The thermal decomposition of poly(catechin)-laccase was as follows: first stage of decomposition temperature 76 °C, mass loss 6.5%; the second stage of decomposition temperature 150–650 °C and mass loss of the sample 22.9%. The first step of decomposition of poly(catechin)-HRP occurred around 65 °C, the mass loss was 11.4%. The second stage of decomposition was found in the temperature range of 150–650 °C. The second stage was accompanied by a mass loss of the sample of 42.7%. The lower initial decomposition temperature of poly(catechin)-HRP may have been due to the lower molecular mass of the sample (tested by GPC) as well as the presence of shorter oligomer chains or polymerization residue. Table 2 lists the values of the temperatures at which the mass loss of the poly(catechin)-laccase and poly(catechin)-HRP samples occurred. It was found that the degradation of poly(catechin)-laccase and poly(catechin)-HRP started at a lower temperature than that of catechin (T10 poly(catechin)-laccase = 222.5 °C; T10 poly(catechin)-HRP = 95.8 °C; T10 catechin = 248 °C). Similar to the previously described flavonoids, the temperature of the final decomposition of poly(catechin)-laccase T45 was 264 °C higher than value for the monomeric catechin. The value of the final thermal decomposition temperature T55 of poly(catechin)-HRP was 81 °C higher than the T55 of the monomeric catechin.
Among the analysed polymeric flavonoids, the highest thermal stability was demonstrated by the oligomeric forms of catechin, especially the poly(catechin)-laccase sample. According to the literature data, compounds of plant origin from the group of polyphenols showed good charring properties. Tannins in particular had excellent charring capacity, therefore they have been proposed for flame-retardant applications [40,41,42]. Condensed tannins (proanthocyanidins, polyflavonoid tannins, catechol-type tannins, pyrocatecollic type tannins, non-hydrolyzable tannins or flavolans) are polymers formed by the condensation of flavans [43]. Catechin and epicatechin are the building blocks of the proanthocyanidins, a type of condensed tannin [44]. Due to the charring ability of the condensed flavan-3-ol [38], the polymeric flavonoids poly(catechin)-laccase and poly(rutin)-HRP showed particularly good thermal stability (TG) and good oxidation resistance (DSC). Probably, formation of a char layer on the surface during thermal degradation protected the inside of the samples against the destructive effects of high temperature and oxidation, improving their thermal stability and resistance to oxidation.
In the case of polymeric samples of quercetin and catechin, it was found that poly(flavonoids) obtained by reaction with the laccase enzyme had better thermal stability. This may be related to the higher molar mass of these samples compared to poly(flavonoids)-HRP, as shown by GPC chromatography. The greater molar mass and greater degree of cross-linking of poly(quercetin)-laccase and poly(catechin)-laccase samples could result in better thermal stability.
Oligomeric rutin samples obtained with laccase and HRP had a similar course of thermal degradation. As shown by GPC analysis, poly(rutin)-HRP was the only sample of the materials obtained in the polymerization reaction with horseradish peroxidase to have a molecular mass within the chromatograph assay range. The similar thermal behaviour of poly(rutin-HRP and poly(rutin)-laccase could have been influenced by the similar length of oligomer chains, while the poly(rutin)-HRP sample was characterized by greater molar mass heterogeneity (greater PDI), which may explain the lower initial thermal degradation temperature. Low molecular mass fractions could decompose at a lower temperature.
The multi-stage decomposition of poly(flavonoids) may be related to the decomposition of the remaining monomers, oligomers with a lower molecular mass, and then larger oligomers. In addition, degradation of substrate residues after polymerization (e.g. enzymes) cannot be ruled out. In addition, the stepwise degradation of flavonoids and poly(flavonoids) may also be related to their physical state. Amorphous fractions may be more susceptible to thermal degradation than crystalline fractions and may decompose earlier than crystalline fractions. The assessment of the crystallinity of the samples and the correlation of the content of the crystalline phase with thermal decomposition requires further research, which is planned in the future.
Antioxidant and antimicrobial properties
The next step was to analyse the antioxidant and antimicrobial properties of flavonoids and poly(flavonoids). The antioxidant activity was determined as the ability to reduce free radicals, ABTS and DPPH, as well as the ability to reduce transition metal ions iron (FRAP test) and copper (CUPRAC test). According to the literature data, the anti-radical activity of flavonoids depends on their ability to resist the oxidizing effect of reactive free radicals [45]. The most important structural elements of flavonoids that provide an effective anti-radical activity are the o-dihydroxy group in the B ring, the C2=C3 double bond connected to the C4=O group in the C ring and the hydroxyl groups in the C3 and C5 ring positions [6, 46]. Table 3 summarizes the antioxidant properties of quercetin, rutin, catechins and their polymeric forms.
From the analysis of antioxidant properties, measured as the ability to reduce free radicals and the ability to reduce metal ions, it is difficult to determine a clear tendency in the behaviour of polymeric flavonoids. A clear tendency to change the antioxidant properties was found only in the case of polymeric forms of catechin obtained with both laccase and HRP. All oligomeric catechin samples had a lower ability to reduce ABTS and DPPH free radicals, as well as a lower ability to reduce transient iron and copper ions. The lower antioxidant activity of the polymeric catechin could be due to the strong cross-linking of oligomeric chains (formation of new catechol-catechol bonds between monomers), due to which the structural elements providing antioxidant activity (including OH functional groups and double bonds) had a spherically limited possibility of reacting with radicals and metal ions. In contrast to the flavonols in the catechin structure, due to the lack of a carbonyl group in the C4 position and saturation of the heterocyclic ring, electron delocalization between the A and B rings in the resulting phenoxy radical is impossible, so the antioxidant properties of catechin depend to a large extent on the number of hydroxyl groups in the molecule. Due to the involvement of catechin hydroxyl groups in enzymatic polymerization, flavan-3-ol (catechin) oligomers may show less activity in reducing ABTS and DPPH free radicals, as well as less ability to reduce copper and iron transition metal ions.
Different antioxidant properties of polymeric quercetin and rutin compounds were shown by ABTS and DPPH methods. Quercetin and rutin oligomers synthesized in reactions with both laccase and HRP were characterized by a greater ability to reduce free cation radicals. For ABTS, the results were poly(quercetin)-laccase more by about 12%, poly(quercetin)-HRP by about 24%, poly(rutin)-laccase approx. 53% and poly(rutin)-HRP approx. 40% compared to the reference samples). In the case of the DPPH method, the oligomeric quercetin and rutin polymerized in the reaction with the laccase enzyme had a clearly lower ability against DPPH radicals. On the other hand, samples with a lower molar mass (according to GPC) poly(quercetin)-HRP and poly(rutin)-HRP showed a slightly better ability to capture DPPH radicals (values similar to the activity of monomeric samples), possibly due to steric hindrance between the large DPPH radical and the larger polymer synthesized with laccase.
The results of the ability of quercetin and rutin oligomers to reduce copper ions (CUPRAC test) showed a clear tendency. Polymeric forms of quercetin and rutin obtained in reactions with laccase and HRP had better ability to reduce Cu2+ copper ions in relation to the reference flavonoids. Poly(rutin)-laccase and poly(rutin)-HRP also showed a greater ability to reduce Fe3+ iron ions (FRAP test) compared to rutin. Poly(quercetin)-HRP was also more active in the FRAP method, while poly(quercetin)-laccase reduced iron ions to a lesser extent than quercetin.
Flavonol oligomers (quercetin and rutin) had a greater ability to reduce ABTS free radicals and Cu2+ copper ions, due to the increase in the number of active hydroxyl groups in the structure of compounds.
The scientific literature describes a similar lack of unambiguity in the correlation between the polymeric form of flavonoids and their antioxidant activity determined by various methods [23, 30]. Analysing the properties of polymeric forms of rutin, Chebil et al. found that a different source (origin) of the same enzyme used in the polymerization reactions may affect the antioxidant properties of the polymeric flavonoid obtained in these reactions. The lack of an unequivocal trend in the behaviour of polymeric flavonoids was also attributed to various methods of determining the antioxidant activity and the degree of polymerization [30]. Despite these ambiguities, many scientific publications indicated that the polymerization of flavonoids allowed to obtain compounds with better antioxidant capacity [22, 24, 28,29,30]. For this reason, in the future, it is worth conducting research that will contribute to the systematization of knowledge about the antioxidant properties of poly(flavonoids).
Table 4 summarizes the antimicrobial properties of flavonoids and poly(flavonoids). Microbiological tests were performed on poly(flavonoids) synthesized with the laccase enzyme.
As in the case of antioxidant properties, it is also difficult to determine an unequivocal trend for antimicrobial activity. Analysing samples of quercetin and poly(quercetin)-laccase, it was found that the oligomeric form of quercetin had comparable activity to quercetin for Candida albicans, while the ability of the polymer to inhibit the growth of Staphylococus aureus, Bacillus subtilis and Aspergillus Niger microorganisms was lower compared to the monomer. Poly(quercetin)-laccase at a concentration of 10 mg mL–1 had greater activity against Escherichia coli than quercetin. Poly(rutin)-laccase had similar antimicrobial properties to rutin. Only the poly(rutin)-laccase sample at the concentration of 10 mg mL–1 showed a significantly smaller growth inhibition zone for Aspergillus niger than the reference rutin and thus less activity against this fungus. Similar results were obtained for the catechin flavonoid and its oligomeric form. Poly(catechin)-laccase had comparable or slightly less inhibitory capacity for most of the microorganisms tested. The activity of poly(catechin)-laccase at a concentration of 10 mg mL–1 against Staphylococus aureus deserved particular attention. The monomeric catechin did not show any antimicrobial properties against Staphylococus aureus, while the inhibition zone of this bacterium for the poly(catechin)-laccase 10 mg mL–1 sample was 18 mm, which indicated an increase in the oligomer's antibacterial activity. The results obtained for antimicrobial activity were not unequivocal, and the lack of a clear tendency could result from the limited solubility of the preparations (especially oligomer samples) and thus the difficulty of penetration of flavonoids and poly(flavonoids) into the cells of microorganisms.
The results of both antioxidant and antimicrobial activity were dependent on the test used and showed no unequivocal tendency. The lack of unequivocal results could be due to the limited solubility of samples, especially oligomeric ones, which could have led to the results being subject to measurement error and some imperfection. However, the methods of analysing antioxidant and anti-microbiological properties, as well as examining the structure of samples often require the use of specific solvents suitable for the test methodology, which was a certain inconvenience of the analyses performed.
Conclusions
The enzymatic polymerization of three flavonoids, differing in the chemical structure, quercetin (flavonol), its glycosides rutin and catechin (flavan-3-ol) allowed oligomeric forms to be obtained. Depending on the enzyme used during the polymerization, oligomers with different molecular mass were obtained. The poly(flavonoids) synthesized with laccase had a higher molar mass than the oligomeric forms obtained in the reaction with horseradish peroxidase. The molar mass of the oligomers influenced their thermal properties.
A common feature of all oligomeric flavonoids was a higher final oxidation temperature and a higher enthalpy of oxidation (DSC analysis) than for monomeric polyphenols. These results indicated a greater resistance to oxidation of both poly(flavonoid)-laccase and poly(flavonoid)-HRP. The improved resistance to oxidation of the oligomeric forms of quercetin, rutin and catechin could have been influenced by the presence of longer oligomeric chains and the cross-linked structure of poly(flavonoids). The factors mentioned improved the thermal properties of oligomeric flavonoids by limiting the access of heat and oxygen to poly(flavonoid) molecules and slowing their thermal decomposition and oxidation reactions. The results obtained from the DSC analysis corresponded with the molar mass analysis (GPC). Oligomeric flavonoids synthesized by reaction with the HRP enzyme had a very low molecular mass (below the GPC quantification), so their melting point was lower than that of poly(flavonoids)-laccase having longer oligomeric chains. The total enthalpy of melting of the polymeric flavonoids (except for poly(quercetin)-HRP) was higher than that of the reference polyphenols.
Based on the thermogravimetric analysis, it was found that, in the case of oligomeric samples of quercetin, rutin and catechin, the samples obtained in the reaction with the laccase enzyme were characterized by better thermal stability. The higher molar mass of the poly(flavonoid)-laccase samples compared to the poly(flavonoids)-HRP could be the reason for better thermal resistance. Higher molar mass and higher degree of cross-linking of poly(quercetin)-laccase and poly(catechin)-laccase samples could have produced the improvement of thermal stability.
All oligomers obtained in the reaction with laccase and with HPR were characterized by better resistance to thermo-oxidation and higher thermo-oxidation enthalpy, resulting from the greater number of hydroxyl groups in the compound. Higher molecular mass oligomers (lacase synthesis) showed better thermal stability related to stronger cross-linking of monomers with new catechol–catechol bonds. The antioxidant properties of poly (flavonoids) also changed due to the increase in the amount of active hydroxyl groups in the structure of compounds—quercetin and rutin had a greater ability to reduce ABTS free radicals and copper ions. On the other hand, catechin, having a different chemical structure than flavonols (quercetin and rutin), after polymerization showed less activity in reducing ABTS and DPPH radicals and metals copper and iron due to the involvement of catechin hydroxyl groups in enzymatic polymerization. Despite the difficult to determine correlations between the structure of oligomers and their properties, the obtained results extended the current state of knowledge and marked the paths for further research.
References
Kabera JN, Semana E, Mussa AR, He X. Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J Pharm Pharmacol. 2014;2:377–92.
Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, et al. Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33(9):2221–43.
Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47.
Al-Khayri JM, Sahana GR, Nagella P, Joseph BV, Alessa FM, Al-Mssallem MQ. Flavonoids as potential anti-inflammatory molecules: a review. Molecules. 2022;27(9):2901.
Wang TY, Li Q, Bi KS. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J Pharm Sci. 2018;13(1):12–23.
Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure–activity relationships. J Nutr Biochem. 2002;13(10):572–84.
Latos-Brozio M, Masek A. Structure-activity relationships analysis of monomeric and polymeric polyphenols (quercetin, rutin and catechin) obtained by various polymerization methods. Chem Biodivers. 2019;16(12):e1900426.
Amic D, Davidovic-Amic D, Beslo D, Rastija V, Lucic B, Trinajstic N. SAR and QSAR of the antioxidant activity of flavonoids. Curr Med Chem. 2007;14:827–45.
Wu T, Zang X, He M, Pan S, Xu X. Structure–activity relationship of flavonoids on their anti-Escherichia coli activity and inhibition of DNA gyrase. J Agric Food Chem. 2013;61:8185–90.
Castillo J, Benavente-García O, Lorente J, Alcaraz M, Redondo A, Ortuño A, et al. Antioxidant activity and radioprotective effects against chromosomal damage induced in vivo by X-rays of flavan-3-ols (Procyanidins) from grape seeds (Vitis vinifera): comparative study versus other phenolic and organic compounds. J Agric Food Chem. 2000;48:1738–45.
Bastide P, Bos MA, Pourrat A. Procyanidins from Tormentil: fractionation and study of the anti-radical activity towards superoxide anion. Biol Pharm Bull. 1994;17:1613–5.
Kanaze FI, Kokkalou E, Niopas I, Georgarakis M, Stergiou A, Bikiaris D. Thermal analysis study of flavonoid solid dispersions having enhanced solubility. J Therm Anal Calorim. 2006;83:283–90.
Kanaze FI, Kokkalou E, Niopas I, Barmpalexis P, Georgarakis E, Bikiaris D. Dissolution rate and stability study of flavanone aglycones, naringenin and hesperetin, by drug delivery systems based on polyvinylpyrrolidone (PVP) nanodispersions. Drug Dev Ind Pharm. 2010;36(3):292–301.
Kanaze FI, Kokkalou E, Niopas I, Georgarakis M, Stergiou A, Bikiaris D. Dissolution enhancement of flavonoids by solid dispersion in PVP and PEG matrixes: a comparative study. J Appl Polym Sci. 2006;102:460–71.
Kirschweng B, Tátraaljai D, Földes E, Pukánszky B. Natural antioxidants as stabilizers for polymers. Polym Degrad Stab. 2017;145:25–40.
Chaaban H, Ioannou I, Chebil L, Slimane M, Gérardin C, Paris C, et al. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J Food Process Preserv. 2017;41(5):e13203.
Buchner N, Krumbein A, Rohn S, Kroh LW. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun Mass Spectrom. 2006;20(21):3229–35.
Ferreira LMB, Kobelnik M, Regasini LO, Dutra LA, da Silva BV, Ribeiro CA. Synthesis and evaluation of the thermal behavior of flavonoids: thermal decomposition of flavanone and 6-hydroxyflavanone. J Therm Anal Calorim. 2017;127:1605–10.
Latos-Brozio M, Masek A, Piotrowska M. Thermally stable and antimicrobial active poly(Catechin) obtained by reaction with a cross-linking agent. Biomolecules. 2021;11(1):50.
Bruno FF, Trotta A, Fossey S, Nagarajan S, Nagarajan R, Samuelson LA, et al. Enzymatic synthesis and characterization of polyquercetin. J Macromol Sci Pure Appl Chem. 2010;47:1191–6.
Sahiner N. One step poly(rutin) particle preparation as biocolloid and its characterization. Mater Sci Eng C. 2014;44:9–16.
Sahiner N. One step poly(quercetin) particle preparation as biocolloid and its characterization. Colloids Surf A Physicochem Eng. 2014;452:173–80.
Latos-Brozio M, Masek A, Piotrowska M. Polymeric forms of plant flavonoids obtained by enzymatic reactions. Molecules. 2022;27(12):3702.
Latos-Brozio M, Masek A, Piotrowska M. Novel polymeric biomaterial based on naringenin. Materials. 2021;14(9):2142.
Anthoni J, Chebil L, Lionneton F, Magdalou J, Humeau C, Ghoul M. Automated analysis of synthesized oligorutin and oligoesculin by laccase. Can J Chem. 2011;89:964–70.
Hamada S, Kontani M, Hosono H, Ono H, Tanaka T, Ooshima T, et al. Peroxidase-catalyzed generation of catechin oligomers that inhibit glucosyltransferase from Streptococcus sobrinus. FEMS Microbiol Lett. 1996;143:35–40.
Desentis-Mendoza RM, Hernández-Sánchez H, Moreno A, del Rojas C, Chel-Guerrero E, Tamariz J, et al. Enzymatic polymerization of phenolic compounds using laccase and tyrosinase from Ustilago maydis. Biomacromol. 2006;7:1845–54.
Anthoni J, Lionneton F, Wieruszeski J-M, Magdalou J, Engasser J-M, Chebil L et al. Investigation of enzymatic oligomerization of rutin. RJC Rasayan J Chem. 2008;1(4):718–31
Kurisawa M, Chung JE, Uyama H, Kobayashi S. Enzymatic synthesis and antioxidant properties of poly(rutin). Biomacromol. 2003;4:1394–9.
Chebil L, Rhouma G ben, Chekir-Ghedira L, Ghoul M. Enzymatic polymerization of rutin and Esculin and evaluation of the antioxidant capacity of polyrutin and polyesculin. Biotechnology. InTech; 2015.
Hosny M, Rosazza JPN. Novel oxidations of (+)-catechin by horseradish peroxidase and laccase. J Agric Food Chem. 2002;50:5539–45.
Kurisawa M, Chung JE, Kim YJ, Uyama H, Kobayashi S. Amplification of antioxidant activity and xanthine oxidase inhibition of catechin by enzymatic polymerization. Biomacromol. 2003;4:469–71.
Kurisawa M, Chung JE, Uyama H, Kobayashi S. Laccase-catalyzed synthesis and antioxidant property of poly(catechin). Macromol Biosci. 2003;3:758–64.
Sigma-Aldrich. Laccase from Aspergillus sp. Available online: https://www.sigmaaldrich.com/PL/pl/product/sigma/sae0050. Accessed 4 Feb 2022
Sigma-Aldrich. Peroxidase from Horseradish. Available online: https://www.sigmaaldrich.com/PL/pl/product/sigma/p6782. Accessed 4 Feb 2022
Masek A, Chrzescijanska E, Latos M, Zaborski M. Influence of hydroxyl substitution on flavanone antioxidants properties. Food Chem. 2017;215:501–7.
Masek A, Chrzescijanska E, Latos-Brozio M, Zaborski M. Characteristics of juglone (5-hydroxy-1,4,-naphthoquinone) using voltammetry and spectrophotometric methods. Food Chem. 2019;301:125279.
Tomás-Barberán FA, Robins RJ. Proceedings of the phytochemical society of Europe. New York: Oxford University Press; 1997. p. 60–6.
Rabek J. Współczesna wiedza o polimerach. Warsaw: PWN; 2008.
Xia Z, Kiratitanavit W, Facendola P, Thota S, Yu S, Kumar J, et al. Fire resistant polyphenols based on chemical modification of bio-derived tannic acid. Polym Degrad Stab. 2018;153:227–43.
Tributsch H, Fiechter S. The material strategy of fire-resistant tree barks. High performance structures and materials IV. Southampton: WIT Press; 2008. p. 43–52.
Drabble E, Nierenstein M. On the role of phenols, tannic acids, and oxybenzoic acids in cork formation. Biochem J. 1907;2:96-102.1.
Cammack R, Atwood T, Campbell P, Parish H, Smith A, Vella F, et al editors. Oxford dictionary of biochemistry and molecular biology. Oxford: Oxford University Press; 2006.
Rinaldo D, Batista JM, Rodrigues J, Benfatti AC, Rodrigues CM, dos Santos LC, et al. Determination of catechin diastereomers from the leaves of Byrsonima species using chiral HPLC-PAD-CD. Chirality. 2010;22(8):726–33.
Zheng Y-Z, Deng G, Chen D-F, Guo R, Lai R-C. The influence of C2–C3 double bond on the antiradical activity of flavonoid: different mechanisms analysis. Phytochemistry. 2019;157:1–7.
Burda S, Oleszek W. Antioxidant and antiradical activities of flavonoids. J Agric Food Chem. 2001;49:2774–9.
Funding
This research was funded by the National Science Centre, Poland (NCN), Grant Number 2018/31/N/ST8/02565.
Author information
Authors and Affiliations
Contributions
Conceptualization and funding acquisition contributed by ML-B and AM; methodology, validation, writing—original draft preparation, writing—review and editing and visualization contributed by ML-B, AM and MP; supervision contributed by AM; project administration contributed by ML-B. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Latos-Brozio, M., Masek, A. & Piotrowska, M. Effect of enzymatic polymerization on the thermal stability of flavonoids. J Therm Anal Calorim 148, 5357–5374 (2023). https://doi.org/10.1007/s10973-023-12089-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12089-1