Abstract
Waste plastics have been regarded as a chronic environmental threat because of their disposal problem. If we do not find a sustainable technology for waste plastic conversion, it will cover the whole planet and collapse the ecosystem. Waste low-density polyethylene (LDPE) and high-density polyethylene were pyrolyzed over sol-gel derived silica–alumina (ASA) and Ti-doped silica–alumina (ASA + Ti) catalysts. The catalysts were calcined at 500 °C and characterized using different conventional techniques, e.g., XRD, FTIR, TG/DTA, SEM, EDS, and BET. Based on characterization, it can be predicted that the nanoparticles are formed of rings via Si–O–M (Al or Ti) linkages, which improved their thermal stability and catalytic activity. The polymer and catalyst were taken at an optimized ratio of 25 m m−1 in a batch reactor for degradation. The Ti-doped silica–alumina catalyst showed the maximum conversion (96%) and gasoline selectivity (45.33%) at comparatively low temperatures (376 °C). The highest specific surface area, uniform mesoporosity, and bimetallic skeleton of the Ti-doped catalyst helped to achieve the best performance. The obtained liquid product comprised both branched aliphatic and aromatic hydrocarbons. The obtained result proves that the catalytic pyrolysis of polyolefin generally advances through carbonium ions, where the catalyst acts as a Lewis acid and Lewis base consecutively. Moreover, the synthesized catalyst plays a significant role in isomerization, cyclization, and aromatization reactions. Sources of approximately 44.13 MJ of energy can be recovered from 1 kg of postconsumer waste plastic. This waste-to-fuel technology should be commercialized because it is eco-friendly and cost-effective.
Graphical abstract











Similar content being viewed by others
References
Alabi OA, Ologbonjaye KI, Awosolu O, Alalade OE. Public and environmental health effects of plastic wastes disposal: a review. J Toxicol Risk Asses. 2019;5:021. https://doi.org/10.23937/2572-4061.1510021.
Lebreton L, Andrady A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019;5:6. https://doi.org/10.1057/s41599-018-0212-7.
Nielsen TD, Hasselbalch J, Holmberg K, Stripple J. Politics and the plastic crisis: A review throughout the plastic life cycle. WIREs Energy Environ. 2020;9:e360. https://doi.org/10.1002/wene.360.
Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):1–5. https://doi.org/10.1126/sciadv.1700782.
Law KL, Starr N, Siegler TR, Jambeck JR, Mallos NJ, Leonard GH. The United States’ contribution of plastic waste to land and ocean. Sci Adv. 2020;6:eabd0288. https://doi.org/10.1126/sciadv.abd0288.
Rustagi N, Pradhan SK, Singh R. Public health impact of plastics: an overview. Indian J Occup Environ Med. 2011;15(3):100–3. https://doi.org/10.4103/0019-5278.93198.
Thushari GGN, Senevirathna JDM. Plastic pollution in the marine environment. Heliyon. 2020;6(8):e04709. https://doi.org/10.1016/j.heliyon.2020.e04709.
North EJ, Halden RU. Plastics and environmental health: the road ahead. Rev Environ Health. 2013;28(1):1–8. https://doi.org/10.1515/reveh-2012-0030.
Aboulkas A, Harfi KE, Bouadili AE. Thermal degradation behaviors of polyethylene and polypropylene. Part I: pyrolysis kinetics and mechanisms. Energy Convers Manag. 2010;51:1363–9. https://doi.org/10.1016/j.enconman.2009.12.017.
Dubdub I, Al-Yaari M. Pyrolysis of low density polyethylene: kinetic study using TGA data and ANN prediction. Polymers. 2020;12:891. https://doi.org/10.3390/polym12040891.
Han B, Chen Y, Wu Y, Hua D, Chen Z, Feng W, Yang M, Xie Q. Co-pyrolysis behaviors and kinetics of plastics–biomass blends through thermogravimetric analysis. J Therm Anal Calorim. 2014;115:227–35. https://doi.org/10.1007/s10973-013-3228-7.
Rahman M, Roy SC, Hassan MM, Mondal BK, Faruk MO, Rahman MA, Zahiduzzaman M, Sharmin A, Hosen MJ, Afroze M, Khan M, Nasreen Z, Matin MA. Catalytic pyrolysis of waste plastics into liquid hydrocarbon using mesoporous kaolin clay. J Bangladesh Acad Sci. 2020;44(1):1–12. https://doi.org/10.3329/jbas.v44i1.48559.
Miandad R, Barakat MA, Rehan M, Aburiazaiza AS, Ismail IMI, Nizami AS. Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts. Waste Manag. 2017;69:66–78. https://doi.org/10.1016/j.wasman.2017.08.032.
Galadima A, Masudi A, Muraza O. Towards low-temperature catalysts for sustainable fuel from plastic: a review. J Environ Chem Eng. 2021;9(6):106655. https://doi.org/10.1016/j.jece.2021.106655.
Achilias DS, Roupakias C, Megalokonomos P, Lappas A, Antonakou EV. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J Hazard Mater. 2007;149:536–42. https://doi.org/10.1016/j.jhazmat.2007.06.076.
Lopez A, Marco ID, Caballero BM, Laresgoiti MF, Adrados A, Aranzabal A. Catalytic pyrolysis of plastic wastes with two different types of catalytic: ZSM-5 zeolite and red mud. Appl Catal B Environ. 2011;104:211–9. https://doi.org/10.1016/j.apcatb.2011.03.030.
Ojha DK, Vinu R. Resource recovery via catalytic fast pyrolysis of polystyrene using zeolites. J Anal Appl Pyrol. 2015;113:349–59. https://doi.org/10.1016/j.jaap.2015.02.024.
Mondal BK, Islam MN, Hossain ME, Abser MN. Microstructural and mineralogical properties of acid and alkali activated coal fly ash. J Adv Chem Sci. 2019;5(1):612–4. https://doi.org/10.30799/jacs.201.19050102.
Mondal BK, Guha F, Abser MN. Waste plastics-to-fuel using fly ash catalyst. Waste Dispos Sustain Energy. 2021;3:13–9. https://doi.org/10.1007/s42768-020-00058-5.
Fadillah G, Fatimah I, Sahroni I, Musawwa MM, Mahlia TMI, Muraza O. Recent progress in low-cost catalysts for pyrolysis of plastic waste to fuels. Catalysts. 2021;11:837. https://doi.org/10.3390/catal11070837.
IEA, World Energy Outlook 2018, IEA, Paris, 2018; https://doi.org/10.1787/weo-2018-en.
Zaccheria F, Santoro F, Iftitah ED, Ravasio N. Brønsted and Lewis solid acid catalysts in the valorization of citronellal. Catalysts. 2018;8(10):410. https://doi.org/10.3390/catal8100410.
Esposito S. Traditional sol–gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials. 2019;12:668. https://doi.org/10.3390/ma12040668.
Wu X, Shao G, Shen X, Cui S, Wang L. Novel Al2O3–SiO2 composite aerogels with high specific surface area at elevated temperatures with different alumina/silica molar ratios prepared by a non-alkoxide sol-gel method. RSC Adv. 2016;6:5611. https://doi.org/10.1039/c5ra19764c.
Hensen EJM, Poduval DG, Magusin PCMM, Coumans AE, van Veen JAR. Formation of acid sites in amorphous silica–alumina. J Catal. 2010;269:201–18. https://doi.org/10.1016/j.jcat.2009.11.008.
Prado LASA, Sriyai M, Ghislandi M, Barros-Timmons A, Schulte K. Surface modification of alumina nanoparticles with silane coupling agents. J Br Chem Soc. 2010;21(12):2238–45. https://doi.org/10.1590/S0103-50532010001200010.
Thommes M. Physical adsorption characterization of nanoporous materials. J Chem Eng Technol. 2010;82(7):1059–73. https://doi.org/10.1002/cite.201000064.
Temuujin J, Burmaa G, Amgalan J, Okada K, Jadambaa T, MacKenzie KJD. Preparation of porous silica from mechanically activated kaolinite. J Porous Mater. 2001;8:233–8. https://doi.org/10.1023/A:1012244924490.
Ishihara A, Negura H, Hashimoto T, Nasu H. Catalytic properties of amorphous silica-alumina prepared using malic acid as a matrix in catalytic cracking of n-dodecane. Appl Catal A. 2010;388:68–76. https://doi.org/10.1016/j.apcata.2010.08.027.
Maldonado CS, Rosa JRDL, Ortiz CJL, Ramirez AH, Barraza FFC, Valente JS. Low concentration Fe-doped alumina catalysts using sol–gel and impregnation methods: the synthesis, characterization and catalytic performance during the combustion of trichloroethylene. Materials. 2014;7:2062–86. https://doi.org/10.3390/ma7032062.
Bernardona C, Osmana MB, Laugel G, Louis B, Pale P. Acidity versus metal-induced Lewis acidity in zeolites for Friedel-crafts acylation. C R Chim. 2017;20(1):20–9. https://doi.org/10.1016/j.crci.2016.03.008.
Akubo K, Nahil MA, Williams PT. Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. J Energy Inst. 2019;92:195–202. https://doi.org/10.1016/j.joei.2017.10.009.
Carati A, Ferraris G, Guidotti M, Moretti G, Psaro R, Rizzo C. Preparation and characterization of mesoporous silica–alumina and silica–titania with a narrow pore size distribution. Catal Today. 2003;77:315–23. https://doi.org/10.1016/S0920-5861(02)00376-0.
Aguado J, Sotelo JL, Serrano DP, Calles JA, Escola JM. Catalytic conversion of polyolefins into liquid fuels over MCM-41: comparison with ZSM-5 and amorphous SiO2−Al2O3. Energy Fuels. 1997;11:1225–31. https://doi.org/10.1021/ef970055v.
Saber O, Gobara HM. Optimization of silica content in alumina–silica nanocomposites to achieve high catalytic dehydrogenation activity of supported Pt catalyst. Egypt J Petrol. 2014;23(4):445–54. https://doi.org/10.1016/j.ejpe.2014.11.001.
Wu X, Shao G, Cui S, Wang L, Shen X. Synthesis of a novel Al2O3–SiO2 composite aerogel with a high specific surface area at elevated temperatures using inexpensive inorganic salt of aluminum. Ceram Int. 2014;42(1):874–82. https://doi.org/10.1016/j.ceramint.2015.09.012.
Agliullin MR, Talzi VP, Filippova NA, Bikbaeva VR, Bubennov SV, Prosochkina TR, Grigorieva NG, Narender N, Kutepov BI. Two-step sol-gel synthesis of mesoporous aluminosilicates: highly efficient catalysts for the preparation of 3,5-dialkylpyridines. Appl Petrochem Res. 2018;8:141–51. https://doi.org/10.1007/s13203-018-0202-0.
Lee JW, Jang YI, Park WS, Kim SW, Lee BJ. Photocatalytic and pozzolanic properties of nano-SiO2/Al2O3–TiO2 powder for functional mortar. Materials. 2019;12:1037. https://doi.org/10.3390/ma12071037.
Lotus AF, Feaver RK, Britton LA, Bender ET, Perhay DA, Stojilovic N, Ramsier RD, Chase GG. Characterization of TiO2–Al2O3 composite fibers formed by electrospinning a sol-gel and polymer mixture. Mater Sci Eng B. 2010;167:55–9. https://doi.org/10.1016/j.mseb.2010.01.027.
Ma Z, Chen W, Hu Z, Pan X, Dong G, Zhou S, Peng M, Li Y, Liao C, Xiao Q, Qiu J. Flexible and thermally stable SiO2–TiO2 composite micro fibers with hierarchical nano-heterostructure. RSC Adv. 2013;3:20132. https://doi.org/10.1039/c3ra43049a.
Roy J, Das S, Maitra S. Mullitization of manganese-doped aluminosilicate diphasic gel. J Ceram Sci Technol. 2014;59(4):299–308. https://doi.org/10.4416/JCST2014-00016.
Nampi PP, Moothetty P, Berry FJ, Mortimerc M, Warrier KG. Aluminosilicates with varying alumina–silica ratios: synthesis via a hybrid sol–gel route and structural characterization. Dalton Trans. 2010;39:5101–7. https://doi.org/10.1039/C001219J.
Wu X, Li W, Shao G, Shen X, Cui S, Zhou J, Wei Y, Chen X. Investigation on the textural and structural evolution of the novel crack-free equimolar Al2O3–SiO2–TiO2 ternary aerogel during thermal treatment. Ceram Int. 2017;43(5):4188–96. https://doi.org/10.1016/j.ceramint.2016.12.044.
Murashkevich AN, Lavitskaya AS, Barannikova TI, Zharskii IM. Infrared absorption spectra and structure of TiO2–SiO2 composites. J Appl Spectrosc. 2008;75:730. https://doi.org/10.1007/s10812-008-9097-3.
Riazian M. Nanostructural characterization, and lattice strain of TiO2–Al2O3–SiO2 coating on glass and SI(100) substrates. J Chil Chem Soc. 2016;61(2):2870–7. https://doi.org/10.4067/S0717-97072016000200005.
Karhu M, Lagerbom J, Reponen PK, Ismailov A, Levanen E. Reaction heat utilization in aluminosilicate-based ceramics synthesis and sintering. J Cervalueci Technol. 2017;08(01):101–12. https://doi.org/10.4416/JCST2016-00094.
Song K, Kim W, Suh C-Y, Bang J-H, Ahn J-W. Preparation of mullite–silica composites using silica–rich monophasic precursor obtained as a byproduct of mineral carbonation of blast-furnace slag. Minerals. 2018;8(5):219. https://doi.org/10.3390/min8050219.
Mozgawa W, Fojud Z, Handke M, Jurga S. MAS NMR and FTIR spectra of framework aluminosilicates. J Mole Struct. 2002;614:281–7. https://doi.org/10.1016/S0022-2860(02)00262-4.
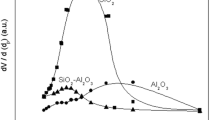
Sing KSW, Williams RT. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt Sci Technol. 2004;22(10):773–82. https://doi.org/10.1260/0263617053499032.
Thommes M, Kaneko K, Neimark AV, Olivier JP, Reinoso FR, Rouquerol J, Sing KSW. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem. 2015;87(9):1051–69. https://doi.org/10.1515/pac-2014-1117.
Wang R, Sang S, Zhu D, Liu S, Yu K. Pore characteristics and controlling factors of the lower Cambrian heating formation shale in northeast Jiangxi. China Energ Explor Exploit. 2018;36(1):43–65. https://doi.org/10.1177/0144598717723814.
Alothman ZA. A review: fundamental aspects of silicate mesoporous materials. Materials. 2012;5:2874–902. https://doi.org/10.3390/ma5122874.
Miandad R, Barakat MA, Aburiazaizaa AS, Rehan M, Nizami AS. Catalytic pyrolysis of plastic waste: a review. Process Saf Environ Prot. 2016;102:822–38. https://doi.org/10.1016/j.psep.2016.06.022.
Ariga K, Vinu A, Yamauchi Y, Ji Q, Hill JP. Nanoarchitectonics for mesoporous materials. Bull Chem Soc Jpn. 2012;85(1):1–32. https://doi.org/10.1246/bcsj.20110162.
Li K, Lee SW, Yuan G, Lei J, Lin S, Weerachanchai P, Yang Y, Wang JY. Investigation into the catalytic activity of microporous and mesoporous catalysts in the pyrolysis of waste polyethylene and polypropylene mixture. Energies. 2016;9:431. https://doi.org/10.3390/en9060431.
Forni L. Comparison of the methods for the determination of surface acidity of solid catalysts. Catal Rev Sci Eng. 2006;8(1):65–115. https://doi.org/10.1080/01614947408071857.
Sivagami K, Kumar KV, Tamizhdurai P, Govindarajan D, Kumar M, Nambi I. Conversion of plastic waste into fuel oil using zeolite catalysts in a bench-scale pyrolysis reactor. RSC Adv. 2022;12:7612–20. https://doi.org/10.1039/D1RA08673A.
Gopinath S, Devan PK, Pitchandi K. Production of pyrolytic oil from ULDP plastics using silica–alumina catalyst and used as fuel for DI diesel engine. RSC Adv. 2020;10:37266–79. https://doi.org/10.1039/D0RA07073D.
Alqarni AO, Nabi RAU, Althobiani F, Naz MY, Shukrullah S, Khawaja HA, Bou-Rabee MA, Gommosani ME, Abdushkour H, Irfan M, Mahnashi MH. Statistical optimization of pyrolysis process for thermal destruction of plastic waste based on temperature-dependent activation energies and pre-exponential factors. Processes. 2022;10:1559. https://doi.org/10.3390/pr10081559.
Eimontas J, Striugas N, Abdelnaby MA, Yousef S. Catalytic pyrolysis kinetic behavior and TG-FTIR-GC-MS analysis of metallized food packaging plastics with different concentrations of ZSM-5 zeolite catalyst. Polymers. 2021;13(5):702. https://doi.org/10.3390/polym13050702.
Ioelovich M. Energy potential of natural, synthetic polymers and waste materials—a review. Academ J Polym Sci. 2018;1(1):09–23. https://doi.org/10.19080/AJOP.2018.01.555553.
Carvill J. Thermodynamics and heat transfer. Mechanical Engineer's data handbook. 1993;102–145. https://doi.org/10.1016/B978-0-08-051135-1.50008-X.
Panda AK, Singh RK, Mishra DK. Thermolysis of waste plastics to liquid fuel a suitable method for plastic waste management and manufacture of value added products—a world prospective. Renew Sust Energ Rev. 2010;14:233–48. https://doi.org/10.1016/j.rser.2009.07.005.
Verma R, Vinoda KS, Papireddy M, Gowda ANS. Toxic pollutants from plastic waste—a review. Procedia Environ Sci. 2016;35:701–8. https://doi.org/10.1016/j.proenv.2016.07.069.
Rowat SC. Incinerator toxic emissions: a brief summary of human health effects with a note on regulatory control. Med Hypotheses. 1999;52(5):389–96. https://doi.org/10.1054/mehy.1994.0675.
Buekens AG, Huang H. Catalytic plastics cracking for recovery of gasoline-range hydrocarbons from municipal plastic wastes. Resour Conserv Recycl. 1998;23:163–81. https://doi.org/10.1016/S0921-3449(98)00025-1.
Wang Y, Cheng L, Gu J, Zhang Y, Wu J, Yuan H, Chen Y. Catalytic pyrolysis of polyethylene for the selective production of monocyclic aromatics over the zinc-loaded ZSM-5 catalyst. ACS Omega. 2022;7(3):2752–65. https://doi.org/10.1021/acsomega.1c05401.
Gebre SH, Sendeku MG, Bahri M. Recent trends in the pyrolysis of non-degradable waste plastics. ChemistryOpen. 2021;10(12):1202–26. https://doi.org/10.1002/open.202100184.
Miskolczi N, Bartha L, Deak G. Thermal degradation of polyethylene and polystyrene from the packaging industry over different catalysts into fuel-like feedstocks. Polym Degrad Stabil. 2006;91(3):517–26. https://doi.org/10.1016/j.polymdegradstab.2005.01.056.
Funding
No endowment had been received from any donator in the public, commercial, or specific grant commission for this investigation.
Author information
Authors and Affiliations
Contributions
BKM contributed to conceptualization, visualization, methodology, investigation, software, data curation, and writing—original draft preparation. FG contributed to writing—reviewing and editing. MNA contributed to supervision and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mondal, B.K., Guha, F. & Abser, M.N. Sol-gel derived Ti-doped mesoporous silica–alumina: an efficient catalyst to recover energy sources from environmental hazard waste plastics. J Therm Anal Calorim 148, 5257–5270 (2023). https://doi.org/10.1007/s10973-023-12059-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12059-7