Abstract
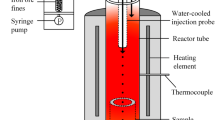
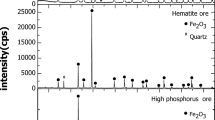
Reduction behavior of commercial natural hematite with respect to degrees of particle packing was studied using a thermogravimetric analysis (TGA) approach. Understanding of temporal mass variations of oxygen carriers such as hematite during reduction is critical to enabling successful industrial applications including ironmaking and chemical looping combustion processes. Gravimetric evolutions of carrier particles as a whole are influenced by how individual particles are physically configured in relation to a reaction gas, especially when the particles are stacked on top of each other, leaving bottom particles less reacted or in some cases unreacted in a static environment. This work reports effects of particle stacking on reduction behaviors of commercial natural hematite isothermally at 800 °C in 10 CO–90% N2 gas environment. Current results indicated, mass evolution kinetics were slowed with more initial mass as stacking layers tended to decrease the available surface area to mass ratio with depth and impeded gas diffusion to particles at deeper locations. Post-SEM analysis revealed an interesting phenomenon where the commercial natural hematite particles subjected to the present experimental approach resulted in a reversed reduction sequence; more reduced phase (metallic iron) was produced inside surrounded by less reduced phase (wüstite). The present work suggested the design of reactors may benefit by considering how the particles are arranged in the reactors.











Similar content being viewed by others
References
Bui M, Adjiman CS, Bardow A, Anthony EJ, Boston A, Brown S, Fennell PS, Fuss S, Galindo A, Hackett LA, Hallett JP. Carbon capture and storage (CCS): the way forward. Energy Environ Sci. 2018;11(5):1062–176.
Rajabi M, Mehrpooya M, Haibo Z, Huang Z. Chemical looping technology in CHP (combined heat and power) and CCHP (combined cooling heating and power) systems: a critical review. Appl Energy. 2019;1(253):113544.
Wang P, Means N, Shekhawat D, Berry D, Massoudi M. Chemical-looping combustion and gasification of coals and oxygen carrier development: a brief review. Energies. 2015;8(10):10605–35.
Zeng L, Luo S, Sridhar D, Fan LS. Chemical looping processes—particle characterization, ionic diffusion-reaction mechanism and reactor engineering. Rev Chem Eng. 2012;28(1):1–42.
Yu Z, Yang Y, Yang S, Zhang Q, Zhao J, Fang Y, Hao X, Guan G. Iron-based oxygen carriers in chemical looping conversions: a review. Carbon Resour Convers. 2019;2(1):23–34.
Haider SK, Azimi G, Duan L, Anthony EJ, Patchigolla K, Oakey JE, Leion H, Mattisson T, Lyngfelt A. Enhancing properties of iron and manganese ores as oxygen carriers for chemical looping processes by dry impregnation. Appl Energy. 2016;1(163):41–50.
Piotrowski K, Mondal K, Wiltowski T, Dydo P, Rizeg G. Topochemical approach of kinetics of the reduction of hematite to wüstite. Chem Eng J. 2007;131(1–3):73–82.
Monazam ER, Breault RW, Siriwardane R. Kinetics of magnetite (Fe3O4) oxidation to hematite (Fe2O3) in air for chemical looping combustion. Ind Eng Chem Res. 2014;53(34):13320–8.
Jiang S, Shen L, Wu J, Yan J, Song T. The investigations of hematite-CuO oxygen carrier in chemical looping combustion. Chem Eng J. 2017;1(317):132–42.
Siriwardane R, Tian H, Miller D, Richards G. Fluidized bed testing of commercially prepared MgO-promoted hematite and CuO–Fe2O3 mixed metal oxide oxygen carriers for methane and coal chemical looping combustion. Appl Energy. 2015;1(157):348–57.
Ma Z, Zhang S, Xiao R. Insights into the relationship between microstructural evolution and deactivation of Al2O3 supported Fe2O3 oxygen carrier in chemical looping combustion. Energy Convers Manage. 2019;15(188):429–37.
Cabello A, Abad A, García-Labiano F, Gayán P, de Diego LF, Adánez J. Kinetic determination of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier for use in gas-fueled chemical looping combustion. Chem Eng J. 2014;15(258):265–80.
Tijani MM, Aqsha A, Mahinpey N. Synthesis and study of metal-based oxygen carriers (Cu Co, Fe, Ni) and their interaction with supported metal oxides (Al2O3, CeO2, TiO2, ZrO2) in a chemical looping combustion system. Energy. 2017;1(138):873–82.
Ma S, Chen S, Soomro A, Xiang W. Effects of CeO2, ZrO2, and Al2O3 supports on iron oxygen carrier for chemical looping hydrogen generation. Energy Fuels. 2017;31(8):8001–13.
Monazam ER, Breault RW, Siriwardane R. Reduction of hematite (Fe2O3) to wüstite (FeO) by carbon monoxide (CO) for chemical looping combustion. Chem Eng J. 2014;15(242):204–10.
Riley J, Siriwardane R, Tian H, Benincosa W, Poston J. Experimental and kinetic analysis for particle scale modeling of a CuO-Fe2O3-Al2O3 oxygen carrier during reduction with H2 in chemical looping combustion applications. Appl Energy. 2018;15(228):1515–30.
Norberg ST, Azimi G, Hull S, Leion H. In situ neutron powder diffraction study of the reaction M2O3↔M3O4↔MO, M=(Fe0.2Mn0.8): implications for chemical looping with oxygen uncoupling. CrystEngComm. 2016;18(29):5537–46.
Nakano A, Nakano J, Bennett J. Real time morphology changes of a single natural hematite particle during cyclic variations in oxygen partial pressures. National Energy Technology Laboratory (NETL), Pittsburgh, PA, Morgantown, WV (United States); 2019 May 7.
Nakano A, Nakano J, Bennett J. Real-time high temperature investigations of an individual natural hematite ore particle for chemical looping oxygen exchange. Appl Energy. 2020;15(268):114926.
Yu J, Han Y, Li Y, Gao P, Li W. Mechanism and kinetics of the reduction of hematite to magnetite with CO–CO2 in a micro-fluidized bed. Minerals. 2017;7(11):209.
Chen H, Zheng Z, Chen Z, Bi XT. Reduction of hematite (Fe2O3) to metallic iron (Fe) by CO in a micro fluidized bed reaction analyzer: a multistep kinetics study. powder technol. 2017;1(316):410–20.
Bogdandy LV, Engell HJ. The reduction of iron ore. 1st ed. New York: Springer-Verlag; 1971.
Piotrowski K, Mondal K, Lorethova H, Stonawski L, Szymański T, Wiltowski T. Effect of gas composition on the kinetics of iron oxide reduction in a hydrogen production process. Int J Hydrogen Energy. 2005;30(15):1543–54.
Qin L, Majumder A, Fan JA, Kopechek D, Fan LS. Evolution of nanoscale morphology in single and binary metal oxide microparticles during reduction and oxidation processes. J Mater Chem A. 2014;2(41):17511–20.
Ma Z, Xiao R, Chen L. Redox reaction induced morphology and microstructure evolution of iron oxide in chemical looping process. Energy Convers Manage. 2018;15(168):288–95.
Acknowledgements
Authors acknowledge Mr. Hugh Thomas (NETL) for TGA experimental. This research was performed in part by an appointment to the U.S. Department of Energy (DOE) Postgraduate Research Program at the National Energy Technology Laboratory (NETL) administered by the Oak Ridge Institute for Science and Education (ORISE).
Funding
This project was funded by the Department of Energy, National Energy Technology Laboratory an agency of the US Government, through a support contract. Neither the US Government nor any agency thereof, nor any of its employees, nor the support contractor, nor any of their employees, makes any warranty, expressor implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the US Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the US Government or any agency thereof.
Author information
Authors and Affiliations
Contributions
The study conception and design were performed by Jinichiro Nakano, Anna Nakano and James Bennett. Material preparation and data collection were performed by Jack Widmer. Data analysis was performed by Jinichiro Nakano, Jack Widmer and Anna Nakano. The first draft of the manuscript was written by Jack Widmer and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Widmer, J., Nakano, J., Nakano, A. et al. Particle packing effect on natural hematite thermal reduction for industrial applications. J Therm Anal Calorim 148, 3273–3281 (2023). https://doi.org/10.1007/s10973-023-12010-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12010-w