Abstract
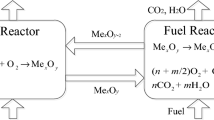
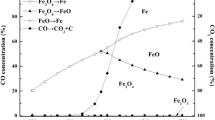
With the increasing demands on syngas quality during chemical looping gasification, investigations on oxygen carriers are becoming more and more significant. A new double-effect oxygen carrier is proposed to further promote the gasification process and capture the main by-product (CO2), which will improve the gasification rate and the quality of the syngas. Three kinds of Cu–Mn composite oxygen carriers modified by CaO with different content were prepared by mechanical mixing. The phase composition and surface morphology of the double-effect oxygen carriers before and after reactions were analyzed, which demonstrated the stability of reactions. Based on thermodynamic experiments, the double-effect oxygen carriers reacted in specific atmospheres to release O2, adsorb CO2, desorb CO2 and absorb O2. By changing the heating rate, reaction temperature and mass ratio of substances in the double-effect oxygen carrier, it can be concluded that increasing the heating rate can accelerate the reaction to some extent. The optimal temperature range for the double-effect oxygen carrier to release O2 and adsorb CO2 is 700–800 °C. To obtain pure CO2, the optimal temperature range for O2 absorption is 700–850 °C and for CO2 desorption is 850–950 °C. The addition of CaO can improve the O2 release velocity and O2 absorption velocity of the active phase of the double-effect oxygen carrier. The kinetic models of the O2 release, CO2 adsorption, CO2 desorption and O2 absorption reactions were established by the iso-conversional method. The O2 release reaction conforms to the one-dimensional diffusion model; the CO2 adsorption reaction conforms to the chemical reaction model (n = 3); the CO2 desorption reaction conforms to the one-dimensional diffusion model; and the O2 absorption reaction conforms to the spherical three-dimensional diffusion model.













Similar content being viewed by others
References
Sansaniwal SK, Rosen MA, Tyagi SK. Global challenges in the sustainable development of biomass gasification: an overview. Renew Sust Energ Rev. 2017;80:23–43.
Moradian JM, Fang Z, Yong Y. Recent advances on biomass-fueled microbial fuel cell. Bioresour Bioprocess. 2021;8(1):14.
Liu W, Li W, Jiang H, Yu H. Fates of chemical elements in biomass during its pyrolysis. Chem Rev. 2017;117(9):6367–98.
Guo Q, Hu X, Liu Y, Jia W, Yang M, Wu M, et al. Coal chemical-looping gasification of Ca-based oxygen carriers decorated by CaO. Powder Technol. 2015;275:60–8.
Hu Q, Shen Y, Chew J, Ge T, Wang C. Chemical looping gasification of biomass with Fe2O3/CaO as the oxygen carrier for hydrogen-enriched syngas production. Chem Eng J. 2020;379:122346.
Hu J, Zhang T, Zhang Q, Yan X, Zhao S, Dang J, Wang W. Application of calcium oxide/ferric oxide composite oxygen carrier for corn straw chemical looping gasification. Bioresour Technol. 2021;330:254–70.
Wang Y, Niu P, Zhao H. Chemical looping gasification of coal using calcium ferrites as oxygen carrier. Fuel Process Technol. 2019;192:75–86.
Yang J, Ma L, Yang J, Guo Z, Liu H, Zhang W. Chemical looping gasification of phosphogypsum as an oxygen carrier: the Ca and S migration mechanism using the DFT method. Sci Total Environ. 2019;689:854–64.
Guo L, Zhang Y, Yi W, Xin Z, Li Z, Zhang Z, et al. Synthesis and characterization of micro-spherical tungsten-molybdenum alloy particles using spray drying combined with microwave assisted calcination process. Int J Refract Met H. 2019;78:45–50.
Hameed Z, Aslam M, Khan Z, Maqsood K, Atabani AE, Ghauri M, et al. Gasification of municipal solid waste blends with biomass for energy production and resources recovery: current status, hybrid technologies and innovative prospects. Renew Sust Energ Rev. 2021;136:110375.
Zhu X, Zhang J, Yan J, Shen L. Characteristic evaluation and process simulation of CuFe2O4 as oxygen carriers in coal chemical looping gasification. ACS Omega. 2021;6(7):4783–92.
Wang K, Yu Q, Qin Q, Hou L, Duan W. Thermodynamic analysis of syngas generation from biomass using chemical looping gasification method. Int J Hydrogen Energy. 2016;41(24):10346–53.
Huang Z, He F, Zheng A, Zhao K, Chang S, Zhao Z, Li H. Synthesis gas production from biomass gasification using steam coupling with natural hematite as oxygen carrier. Energy. 2013;53:244–51.
Shen T, Ge H, Shen L. Characterization of combined Fe–Cu oxides as oxygen carrier in chemical looping gasification of biomass. Int J Greenh Gas Control. 2018;75:63–73.
Ren T, An M, Hu X, Ma J, Guo Q. Development of inexpensive perovskite Mn-based oxygen carriers using the waste manganese sand for chemical looping gasification. Int J Energ Res. 2020;45(2):2416–31.
Ryden M, Cleverstam E, Johansson M, Lyngfelt A, Mattisson T. Fe2O3 on Ce-, Ca-, or Mg-stabilized ZrO2 as oxygen carrier for chemical-looping combustion using NiO as additive. Aiche J. 2010;56(8):2211–20.
Zhang S, Xiao R, Yang Y, Chen L. CO2 capture and desulfurization in chemical looping combustion of coal with a CaSO4 oxygen carrier. Chem Eng Technol. 2013;36(9):1469–78.
Gayan P, Pans MA, Ortiz M, Abad A, de Diego LF, Garcia-Labiano F, Adanez J. Testing of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier for a SR-CLC system in a continuous CLC unit. Fuel Process Technol. 2012;96(4):37–47.
Dennis JS, Scott SA. In situ gasification of a lignite coal and CO2 separation using chemical looping with a Cu-based oxygen carrier. Fuel. 2010;89(7):1623–40.
Matzen M, Pinkerton J, Wang X, Demirel Y. Use of natural ores as oxygen carriers in chemical looping combustion: a review. Int J Greenh Gas Control. 2017;65:1–14.
Wang X, Xu T, Liu S, Xiao B, Hu Z, Chen Z. CuO supported on manganese ore as an oxygen carrier for chemical looping with oxygen uncoupling (CLOU). Chem Eng J. 2018;343:340–50.
Wang B, Li H, Wang W, Luo C, Mei D, Zhao H. Reaction characteristic investigation of the combined template-method-made CaSO4–Mn3O4 mixed oxygen carrier with lignite. Energ Fuel. 2019;33(9):8954–66.
Zhou H, Wei G, Yi Q, Zhang Z, Zhao Y, Zhang Y, et al. Reactivity investigation on chemical looping gasification of coal with Iron–Manganese based oxygen carrier. Fuel. 2022;307:121772.
Pour NM, Leion H, Ryden M, Mattisson T. Combined Cu/Mn oxides as an oxygen carrier in chemical looping with oxygen uncoupling (CLOU). Energy Fuel. 2013;27(10):6031–9.
Wang T, Gao Y, Liu Y, Song M, Liu J, Guo Q. Core-shell Na2WO4/CuMn2O4 oxygen carrier with high oxygen capacity for chemical looping oxidative dehydrogenation of ethane. Fuel. 2021;303:121286.
Machida H, Ando R, Esaki T, Yamaguchi T, Horizoe H, Kishimoto A, et al. Low temperature swing process for CO2 absorption–desorption using phase separation CO2 capture solvent. Int J Greenh Gas Control. 2018;75:1–7.
Zhang Z, Borhani TN, Olabi AG. Status and perspective of CO2 absorption process. Energy. 2020;205:118057.
Berstad D, Anantharaman R, Neksa P. Low-temperature CO2 capture technologies-applications and potential. Int J Refrig. 2013;36(5):1403–16.
Zhang Z, Chen F, Rezakazemi M, Zhang W, Lu C, Chang H, Quan X. Modeling of a CO2-piperazine-membrane absorption system. Chem Eng Res Des. 2018;131:375–84.
Chang PT, Ng QH, Ahmad AL, Low SC. A critical review on the techno-economic analysis of membrane gas absorption for CO2 capture. Chem Eng Commun. 2021;209(11):1553–69.
Jung WH, Lee J. Economic evaluation for four different solid sorbent processes with heat integration for energy-efficient CO2 capture based on PEI-silica sorbent. Energy. 2022;238:121864.
Slostowski C, Marre S, Dagault P, Babot O, Toupance T, Aymonier C. CeO2 nanopowders as solid sorbents for efficient CO2 capture/release processes. J CO2 Util. 2017;20:52–8.
Wang J, Kang D, Shen B, Sun H, Wu C. Enhanced hydrogen production from catalytic biomass gasification with in-situ CO2 capture. Environ Pollut. 2020;267: 115487.
Gu H, Song G, Niu M, Zhao S, Gao Y, Li F. Sr2CeO4 as a robust high temperature sorbent for CO2 capture with near 100% sorbent conversion efficiency. Chem Eng J. 2022;441:135942.
Peltzer D, Munera J, Cornaglia L, Strumendo M. Characterization of potassium doped Li2ZrO3 based CO2 sorbents: stability properties and CO2 desorption kinetics. Chem Eng J. 2018;336:1–11.
Xie H, Yu Q, Wei M, Duan W, Yao X, Qin Q, Zuo Z. Hydrogen production from steam reforming of simulated bio-oil over Ce-Ni/Co catalyst with in continuous CO2 capture. Int J Hydrog Energy. 2015;40(3):1420–8.
Nityashree N, Manohara GV, Maroto-Valer MM, Garcia S. Advanced high-temperature CO2 sorbents with improved long-term cycling stability. ACS Appl Mater Interfaces. 2020;12(30):33765–74.
Li Z, Cai N, Huang Y, Han H. Synthesis, experimental studies, and analysis of a new calcium-based carbon dioxide absorbent. Energ Fuel. 2005;19(4):1447–52.
Zhu Q, Zeng S, Yu Y. A model to stabilize CO2 uptake capacity during carbonation–calcination cycles and its case of CaO–MgO. Environ Sci Technol. 2017;51(1):552–9.
Song H, Shah K, Doroodchi E, Wall T, Moghtaderi B. Reactivity of Al2O3- or SiO2-supported Cu-, Mn-, and Co-oxygen carriers for chemical looping air separation. Energ Fuel. 2014;28(2):1284–94.
Adanez J, de Diego L, Garcia-Labiano F, Gayan P, Abad A, Palacios JM. Selection of oxygen carriers for chemical-looping combustion. Energ Fuel. 2004;18(2):371–7.
Xu L, Wang J, Li Z, Cai N. Experimental study of cement-supported CuO oxygen carriers in chemical looping with oxygen uncoupling (CLOU). Energ Fuel. 2013;27(3):1522–30.
Arjmand M, Azad AM, Leion H, Lyngfelt A, Mattisson T. Prospects of Al2O3 and MgAl2O4-supported CuO oxygen carriers in chemical-looping combustion (CLC) and chemical-looping with oxygen uncoupling (CLOU). Energ Fuel. 2011;25(11):5493–502.
Pour NM, Leion H, Ryden M, Mattisson T. Combined Cu/Mn oxides as oxygen carrier in chemical-looping with oxygen uncoupling (CLOU). Energy Fuel. 2013;27(10):6031–9.
Wang K, Yu Q, Wu T, Annaland MV, Qin Q. Thermodynamic and kinetic characteristics of a Cu–Mn composite oxygen carrier for low-temperature chemical-looping air separation. Chem Eng J. 2020;393:124792.
Lv P, Xiong Z, Chang J, Wu C, Chen Y, Zhu J. An experimental study on biomass air-steam gasification in a fluidized bed. Bioresour Technol. 2004;95(1):95–101.
Acknowledgements
This research was financially supported by National Natural Science Foundation of China (52276102).
Author information
Authors and Affiliations
Contributions
Kun Wang conceived the idea of the study and analyzed the data; Yunwei Zhang and Xue meng interpreted the results and wrote the paper; all authors discussed the results and revised the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, K., Zhang, Y., Xue, M. et al. Experimental and kinetic investigations of double-effect oxygen carriers for chemical looping gasification. J Therm Anal Calorim 148, 867–882 (2023). https://doi.org/10.1007/s10973-022-11781-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11781-y