Abstract
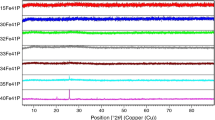
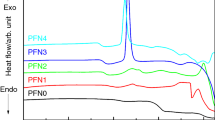
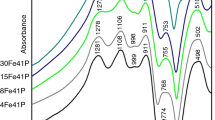
In the present study, effect of europium on the structural and thermal properties of iron phosphate glass was studied and compared with that of pristine iron phosphate glass. Europium-loaded iron phosphate glass [composition-3 mass% Eu2O3-97 mass% (40 mol% Fe2O3-60 mol% P2O5)] was synthesized by conventional melt quench technique, and its amorphous nature was ascertained by X-ray diffraction method. The presence of europium in iron phosphate glass lowered the glass stability and glass forming ability of pristine iron phosphate glass. The presence of europium also resulted in lower specific heat capacity and higher thermal expansion. This is due to the depolymerization of glass network connectivity in the presence of europium, which was further confirmed from the Raman spectra. However, the major network linkage is pyrophosphate as that of pure iron phosphate glass.






Similar content being viewed by others
References
Ojovan MI, Lee WE, Kalmykov SN. Immobilisation of radioactive wastes in glass. In: An Introduction to nuclear waste immobilization. Amsterdam: Elsevier; 2019. p. 319–68. https://doi.org/10.1016/C2017-0-03752-7.
Brow RK, Kim CW, Reis ST. Iron polyphosphate glasses for waste immobilization. Int J Appl Glass Sci. 2020;11:4–14. https://doi.org/10.1111/ijag.13565.
Day DE, Ray CS. A review of iron phosphate glasses and recommendations for vitrifying hanford waste. Idaho National Laboratory United States. 2013. https://doi.org/10.2172/1130550.
Joseph K, Asuvathraman R, Venkata Krishnan R, Ravindran TR, Govindaraj R, Govindan Kutty KV. Iron phosphate glass containing simulated fast reactor waste: characterization and comparison with pristine iron phosphate glass. J Nucl Mater. 2014;452:273–80. https://doi.org/10.1016/j.jnucmat.2014.05.038.
Wang F, Wang Y, Liao Q, Zhang J, Zhao W, Yuan Y, et al. Immobilization of a simulated HLW in phosphate based glasses/glass-ceramics by melt-quenching process. J Non Cryst Solids. 2020;545: 120246. https://doi.org/10.1016/j.jnoncrysol.2020.120246.
Joseph K, Premila M, Amarendra G, Govindan Kutty KV, Sundar CS, Vasudeva Rao PR. Structure of cesium loaded iron phosphate glasses: an infrared and Raman spectroscopy study. J Nucl Mater. 2012;420:49–53. https://doi.org/10.1016/j.jnucmat.2011.09.008.
Joseph K, Ravindran TR, Sudha R, Asuvathraman R. BaO-Fe2O3-P2O5 glasses: understanding the thermal stability. J Nucl Mater. 2019;517:106–12. https://doi.org/10.1016/j.jnucmat.2019.01.046.
Li S, Liu H, Wu F, Chang Z, Yue Y. Effects of alkaline-earth metal oxides on structure and properties of iron phosphate glasses. J Non Cryst Solids. 2016;434:108–14. https://doi.org/10.1016/j.jnoncrysol.2015.12.004.
Lu M, Wang F, Liao Q, Chen K, Qin J, Pan S. FTIR spectra and thermal properties of TiO2-doped iron phosphate glasses. J Mol Struct. 2015;1081:187–92. https://doi.org/10.1016/j.molstruc.2014.10.029.
Šantić A, Moguš-Milanković A, Furić K, Bermanec V, Kim CW, Day DE. Structural properties of Cr2O3-Fe2O3-P2O5 glasses, Part I. J Non Cryst Solids. 2007;353:1070–7. https://doi.org/10.1016/j.jnoncrysol.2006.12.104.
Wang Y, Wang F, Zhou J, Zhu H, Liao Q, Li L. Effect of molybdenum on structural features and thermal properties of iron phosphate glasses and boron-doped iron phosphate glasses. J Alloys Compd. 2020;826: 154225. https://doi.org/10.1016/j.jallcom.2020.154225.
Szreder NA, Lenarciak A, Karczewski J, Gazda M, Barczyński RJ. Impedance studies of phosphate-iron glasses containing niobium and titanium. Procedia Eng. 2014;98:56–61. https://doi.org/10.1016/j.proeng.2014.12.488.
Qian B, Liang X, Yang S, He S, Gao L. Effects of lanthanum addition on the structure and properties of iron phosphate glasses. J Mol Struct. 2012;1027:31–5. https://doi.org/10.1016/j.molstruc.2012.05.078.
Lai YM, Liang XF, Yang SY, Wang JX, Cao LH, Dai B. Raman and FTIR spectra of iron phosphate glasses containing cerium. J Mol Struct. 2011;992:84–8. https://doi.org/10.1016/j.molstruc.2011.02.049.
Lai Y, Liang X, Yin G, Yang S, Wang J, Zhu H. Infrared spectra of iron phosphate glasses with gadolinium oxide. J Mol Struct. 2011;1004:188–92. https://doi.org/10.1016/j.molstruc.2011.08.003.
Govindan Kutty KV, Asuvatharaman R, Krishnaiah MV, Ganesan V, Parthasarathy R, Sai Subalakshmi D, Suhasini B, Srinivas KC, Gopal KA, Kumar PV. Design, fabrication and commissioning of a push rod dilatometer for thermal expansion studies on solids, IGCAR Report number IGC-283;2006
Krishnaiah MV, Asuvathraman R, Joseph K, Suhasini B, Govindan Kutty KV. Drop calorimeter for the measurement of enthalpy increment of solids: design, fabrication and commissioning. IGCAR report number IGC-319;2013
Day DD, Ray CS, Marasinghe K, Karabulut M. An alternative host matrix based on iron phosphate glasses for the vitrification of specialized waste forms. 2000;1–36. Final Report, Project No. 55110, 2000. Available from: http://www.osti.gov/em52/final_reports/55110.pdf.
Hruby A. Evaluation of glass-forming tendency by means of DTA. Czech J Phys B. 1972;22:1187–93. https://doi.org/10.1007/BF01690134.
Weinberg MC. Glass-forming ability and glass stability in simple systems. J Non Cryst Solids. 1994;167:81–8. https://doi.org/10.1016/0022-3093(94)90370-0.
Lu ZP, Liu CT. A new approach to understanding and measuring glass formation in bulk amorphous materials. Intermetallics. 2004;12:1035–43. https://doi.org/10.1016/j.intermet.2004.04.032.
Inoue A, Zhang T, Masumoto T. Glass-forming ability of alloys. J Non Cryst Solids. 1993;156–158:473–80. https://doi.org/10.1016/0022-3093(93)90003-G.
Mondal K, Murty BS. On the parameters to assess the glass forming ability of liquids. J Non Cryst Solids. 2005;351:1366–71. https://doi.org/10.1016/j.jnoncrysol.2005.03.006.
Yuan ZZ, Bao SL, Lu Y, Zhang DP, Yao L. A new criterion for evaluating the glass-forming ability of bulk glass forming alloys. J Alloys Compd. 2008;459:251–60. https://doi.org/10.1016/j.jallcom.2007.05.037.
Du XH, Huang JC, Liu CT, Lu ZP. New criterion of glass forming ability for bulk metallic glasses. J Appl Phys. 2007;101:12–5. https://doi.org/10.1063/1.2718286.
Du XH, Huang JC. New criterion in predicting glass forming ability of various glass-forming systems. Chin Phys B. 2008;17:249–54.
Zhang P, Wei H, Wei X, Long Z, Su X. Evaluation of glass-forming ability for bulk metallic glasses based on characteristic temperatures. J Non Cryst Solids. 2009;355:2183–9. https://doi.org/10.1016/j.jnoncrysol.2009.06.001.
Joseph K, Ghosh S, Govindan Kutty KV, Vasudeva Rao PR. Crystallization kinetics, stability and glass forming ability of iron phosphate and cesium loaded iron phosphate glasses. J Nucl Mater. 2012;426:233–9. https://doi.org/10.1016/j.jnucmat.2012.03.048.
Joseph K, Asuvathraman R, Venkata Krishnan R, Joseph J, Govindan Kutty KV, Vasudeva Rao PR. Investigation of thermal expansion and specific heat of cesium loaded iron phosphate glasses. J Nucl Mater. 2012;429:1–6. https://doi.org/10.1016/j.jnucmat.2012.05.029.
Long Z, Liu W, Zhong M, Zhang Y, Zhao M, Liao G, Chen Z. A new correlation between the characteristics temperature and glass-forming ability for bulk metallic glasses. J Therm Anal Calorim. 2018;132:1645–60. https://doi.org/10.1007/s10973-018-7050-0.
Guo S, Lu ZP, Liu CT. Identify the best glass forming ability criterion. Intermetallics. 2010;18:883–8. https://doi.org/10.1016/j.intermet.2009.12.025.
Lu ZP, Li Y, Ng SC. Reduced glass transition temperature and glass orming ability of bulk glass forming alloys. J Non Cryst Solids. 2000;270:103–14. https://doi.org/10.1016/S0022-3093(00)00064-8.
Louzguine-Luzgin DV, Miracle DB, Louzguina-Luzgina L, Inoue A. Comparative analysis of glass-formation in binary, ternary, and multicomponent alloys. J Appl Phys. 2010;108: 103511. https://doi.org/10.1063/1.3506687.
Liang X, Li H, Wang C, Yu H, Li Z, Yang S. Physical and structural properties of calcium iron phosphate glass doped with rare earth. J Non Cryst Solids. 2014;402:135–40. https://doi.org/10.1016/j.jnoncrysol.2014.05.021.
Muñoz F, Rocherullé J, Ahmed I, Hu L. Phosphate glasses. In: Musgraves JD, Hu J, Calvez L, editors. Springer handbook of glasses. Cham: Springer; 2019. p. 553–94. https://doi.org/10.1007/978-3-319-93728-1_16.
Brow RK. Review: the structure of simple phosphate glasses. J Non Cryst Solids. 2000;263:1–28. https://doi.org/10.1016/S0022-3093(99)00620-1.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Kitheri Joseph], [Soja K Vijay], [R. Raja Madhavan], [Soumee Chakraborty] and [Ashish Jain]. The first draft of the manuscript was written by [Soja K Vijay] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization: [Kitheri Joseph]; Methodology: [Kitheri Joseph], [Soja K Vijay], [R. Raja Madhavan] and [Soumee Chakraborty]; Formal analysis and investigation: [Kitheri Joseph]; Writing—original draft preparation: [Soja K Vijay]; Writing—review and editing: [Ashish Jain], [R. Raja Madhavan] and [Kitheri Joseph].
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vijay, S.K., Madhavan, R.R., Chakraborty, S. et al. Effect of europium on the structural and thermal properties of pristine iron phosphate glass. J Therm Anal Calorim 148, 321–328 (2023). https://doi.org/10.1007/s10973-022-11749-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11749-y