Abstract
Iron phosphate-silicate glasses from P2O5–SiO2–K2O–MgO–CaO–Fe2O3 system were subjected to the thermal and spectroscopic studies in order to gain information about their structure and thermal behavior in the range of glass transition effect. Research includes results obtained via DSC, MIR and DRIFT spectroscopy. Designated values of glass transition temperature and specific heat change slightly increases with Fe2O3 incorporation. Spectra collected during thermal treatment of glasses containing 2 and 30 mol% Fe2O3 exhibited various changes. Fe2O3 addition affected the glass structure by its reinforcement and led to its preservation during thermal treatment. The connection between density, molar volume, oxygen packing density and the chemical composition’s alteration were also established because of the direct dependence of physical properties and the structure. Obtained results supported thermal and spectroscopic studies. Conducted research is considered as a contribution to the knowledge about the family of iron phosphate glasses, which are known from their interesting properties and widely used applications.
Similar content being viewed by others
Introduction
Iron phosphate glasses present outstanding properties enabling them to be used in various applications [1,2,3,4,5,6,7,8,9,10,11,12,13]. As an example, the great storage capacity of high wastes manifested by traditional iron phosphate glasses characterized by molar composition 60P2O5–40Fe2O3 may be mentioned [14]. Concomitantly, little is known about iron phosphate X-ray amorphous materials containing also silicate, potassium, magnesium and calcium ions. These are considered as eco-friendly and can be potentially implemented into the soil without causing any harm in natural environment. It also needs to be mentioned that the additional components incorporation presumably impacts on iron phosphate glasses properties, such as chemical stability and durability, electric and magnetic properties, response to thermal treatment. This effect has never been studied. Thus, there is a lack of information about good qualities exhibited by glasses from P2O5–SiO2–K2O–MgO–CaO–Fe2O3 system. The research conducted to evaluate the influence of additives on phosphate glasses allows to gain invaluable information. It also entails the possibility to tailor the materials properties and discover their new applications. Presented work is a contribution which enables to increase the knowledge about iron phosphate glasses family.
Iron phosphate glasses are commonly characterized by O/P ratio indicating whether orthophosphate, pyrophosphate or metaphosphate units prevail in the structure [15] which also is verifiable via Raman and infrared spectroscopic studies. It is also agreed that the type of additives and their quantitative proportions determine various material properties. Amorphous materials from Fe2O3–P2O5–RnOm system where n = 0, 1, m = 0, 2 and R = Si, K, Ca, Mg were found to be analyzed in cited papers [14, 16,17,18].
Designated value of glass transition temperature (Tg) revealed in iron phosphate glasses with O/P ratio ~ 3.5 modified by incorporation of 5 mol% SiO2 is slightly increased compared to the parent glass [14] and it constitutes 508 °C. Results obtained in [16] indicated that in ternary glass system Fe2O3–P2O5–K2O, substitution of Fe2O3 with K2O leads to decrease of Tg. Authors have designated it as approx. 340 °C for potassium phosphate glass containing 11.5 mol% Fe2O3 and characterized by O/P ~ 3.1 [16]. Along with CaO addition in xCaO-(32 − x)Fe2O3–68P2O5 glass variation of Tg value is more complex: it rises from 548 to 574 °C for sample comprising 16 mol% CaO and decreases up to 539 °C for 32 mol % CaO [17]. The increase of MgO content at the expense of Fe2O3 results in alteration in Tg value from 499 °C to 545 °C in the (40 − x)MgO–xFe2O3–60P2O5 series [18].
In the ternary glasses, the influence of mentioned components on glass transition temperature is varied. The present study allows to shed light on this matter in far more complex P2O5–SiO2–K2O–MgO–CaO–Fe2O3 system. Tg variability was revealed by means of DSC. Moreover, Diffused Reflectance Fourier Transform Infrared Spectroscopy was used to determine the structural changes within the glass transition effect and simultaneously the response of the structure to thermal treatment was registered. The influence of chemical composition on the structure was also analyzed via Fourier transform infrared spectroscopy. Density measurements were carried out in order to establish a precise connection with physical properties and supported the interpretation of thermal and structural studies.
Experimental
The description of preparation of glasses from P2O5–SiO2–K2O–MgO–CaO–Fe2O3 system with quantitative proportion of P2O5:SiO2:K2O and MgO:CaO set as 41:6:6 and 1.5:1 was included in the study presented in [19]. Chemical composition is expressed by Fe/(Fe + Ca + Mg) ratio (Table 1), as only the content of iron, magnesium and calcium ions is varied. It needs to be noted that the notation of each glass contains information about the amount of incorporated iron oxide. For example, 2Fe41P means the 2 mol% Fe2O3 addition.
Thermal stability of the obtained glasses was determined by DSC measurements conducted on Netzsch STA 449 F3 Jupiter 7 operating in the heat flux DSC mode. The temperature and heat calibrations of the instrument were performed using the melting temperatures and melting enthalpies of high-purity materials (Al, Zn, Sn, Au, Ag). The samples (45 mg) were heated in platinum crucibles at 10 °C min−1 in air atmosphere up to 600 °C. The glass transformation temperature Tg determined as the midpoint of the cp changes in the glass transformation region and changes in specific heat (Δcp) accompanying the glass transformation was determined by applying the Netzsch Proteus Thermal Analysis Program (version 5.0.0.).
Density of the samples was measured by helium pycnometry technique using Micromeritics AccuPyc II 1340 Gas Pycnometer apparatus. In this apparatus, helium is pumped to the chamber with the sample, until certain pressure is reached (in this case, 19.50 psi = 1.37 atm). Then, the gas from the chamber with the sample is pumped to another chamber, where its pressure is measured. The volume of the sample is calculated basing on the differences in the pressure of the gas in the both chambers using ideal gas law (helium can be treated as the ideal gas). Knowing volume of the sample and its mass, which is determined before the measurement with an accuracy of 0.0001 g, software is calculating the density of the sample with an accuracy up to 0.0001 g cm−3. Measurement was repeated 30 times to ensure precision. Before the measurement, the sample was purged with helium 70 times to remove impurities and stabilize its temperature and—which implies—volume.
FTIR spectra were collected in room temperature using a Bruker Company Vertex 70v spectrometer in the range of 1400–400 cm−1. 128 scans at the 4 cm−1 resolution were accumulated. Standard KBr pellet method was used in sample preparation. The amount of KBr and appropriately grinded fraction of glass were accurately weighed.
High temperature DRIFTS (Diffused Reflectance Fourier Transform Infrared Spectroscopy) measurements were performed using Bruker Vertex 70v spectrometer equipped with Harrick Scientific “Praying Mantis” DRS attachment and high temperature reaction chamber with KBr windows (HVC-DRP-5). A total of 128 scans were accumulated in the range of 4000–400 cm−1. Scanner velocity was set to 5 kHz along with 4 cm−1 resolution. Each measurement was carried out after 5 min of temperature stabilization.
Fourier transform infrared spectroscopy results obtained at ambient temperature
Figure 1 presents spectra of glasses containing 2, 4, 8, 15 and 30 mol% Fe2O3 recorded at room temperature. Bands have been revealed in the 1400–1200 cm−1, 1200–850 cm−1, 850–650 cm−1, 650–400 cm−1 regions and their assignment is performed.
1400–1200 cm−1
Distinct band at 1281 cm−1 is observed for 2Fe41P glass. Progressive substitution of CaO and MgO by Fe2O3, manifested also in alterations of Fe/(Fe + Ca + Mg) ratio within the range from 0.087 up to 0.782, is associated with the shift of its location to lower wavenumber. Moreover, in 15Fe41P and 30Fe41P glasses its separateness is significantly more influenced. It may be assigned to asymmetric stretching vibrations of two non-bridging oxygen atoms bonded to phosphorus atoms O–P–O in PO2− groups in metaphosphate units [16, 20,21,22]. The aforementioned variation confirms the depolymerizing role of Fe2O3 on the phosphorus-oxygen subnetwork concluded from Raman study [19].
1200–950 cm−1
Spectrum of glass with 2 mol% iron oxide addition is characterized by pronounced band at 1108 cm−1 and minor band at 999 cm−1. Concomitantly, with increase of Fe/(Fe + Ca + Mg) ratio the visible broadening is detected, mainly the band localized at higher wavenumbers (1108 cm−1 → 1064 cm−1) are affected.
According to [23], band at about 1100 cm−1 corresponds to the combination of P–O and Si–O stretching vibration in P–O–P and P–O–Si linkages in metaphosphate units. Following Hudgens and Martin [24], it may be also assigned to the symmetric stretching of PO2 groups. It shifts to lower wavenumbers with increase of Fe/(Fe + CaO + MgO) ratio. Observed alteration in analyzed spectra of iron phosphate-silicate glasses are considered as advanced, what is apparently connected to the strong effect of ferrous and ferric ions especially on phosphorus-oxygen subnetwork, indicated also in [25]. Thus, spectra of 15Fe41P and 30Fe41P glasses are characterized by broaden bands with maximum designated at 1093 cm−1 and 1064 cm−1. The first one may be attributed to asymmetric stretching vibrations vas(P2O7)4− in pyrophosphate units [26, 27]. It is consistent with results obtained from analysis of Raman spectrum of 15Fe41P [19]. The second one may be a corollary of (PO4)3− asymmetric stretching in orthophosphate units [21, 28, 29]. Although the assignment of the band observed in spectrum of 2Fe41P is not definite, the tendency to the network depolymerization is once again revealed.
Bands at about 999 cm−1 are connected to asymmetric [23] or symmetric [16] stretching vibrations in metaphosphate units [23], particularly P–O bonds in the (PO3)2− end chain groups are implied to be involved [22, 30]. Corbridge and Lowe [29] indicated the presence of band located in similar position (approx. 1000 cm−1) as a distinguishing feature of cyclic metaphosphate compounds.
Another possible interpretation of band at about 999 cm−1 is the asymmetric stretching of (PO4)3− anions [31]. It would indicate that in 2Fe41P glass certain amount of isolated phosphate polyhedra is present. These comparatively inconspicuous bands seem to be increasing with gradual iron oxide addition.
950–650 cm−1
The bands in aforementioned region are generally connected to asymmetric and symmetric stretching vibrations of P–O–P linkages [29, 32] in phosphate glasses. The conspicuous upshift observed in the 850–950 cm−1 region is presumably associated with decreasing length of the P–O–P chain [29]. Therefore, due to position at 927 cm−1, the structure of 30Fe41P is characterized by shorter chains and increased number of pyrophosphate units [26, 28, 33]. Simultaneously in 2Fe41P glass, the band at 911 cm−1 indicate that increased number of P–O–P linkages compared to 30Fe41P glass are revealed, and it is another confirmation of the mainly depolymerizing role manifested by iron ions.
Bands located at lower frequencies in this region may be connected to vibrations occurring in linear chains, cyclic metaphosphates and also pyrophosphate links. Glasses with lower iron oxide addition are characterized by broaden band between 774 and 700 cm−1. This absorption bands may be also connected to links in cyclic metaphosphates [29], such as (P2O6)2− which are characterized by two P–O–P linkages [22]. More sharp band at 753 cm−1 is more likely to be corresponding to pyrophosphate units (P2O7)4− within the single P–O–P linkage [22, 33]. Such behavior presented in this region is another proof of gradual distortion of the structure. It is in agreement with interpretation presented in [30] dedicated to the study of vanadium barium phosphate glasses which exhibit a similar behavior.
650–400 cm−1
The low frequency band in the 490–520 cm−1 region is presumably connected to harmonics of bending vibration of O=P–O linkages [32]. It may be also a manifestation of combination of bending vibrations of O–Si–O and O–P–O bonds [23].
Simultaneously, band at 638 cm−1 observed in spectrum of 30Fe41P is located in the characteristic region in MIR spectra of crystalline Fe2O3 [34, 35]. It was pointed out that vibration of Fe–O may be reflected more clearly on the spectra below 300 cm−1 [32], particularly at 230 cm−1 for ferrous and 290 cm−1 for ferric ions. It is also possible, that bands which are due to Fe–O are masked or superimposed with vibration of O=P–O linkages [35]. Nevertheless, a spectrum of 30Fe41P glass is quite distinctive. The conspicuous band at 542 cm−1 may be also connected to O–P–O bending vibrations particularly in pyrophosphate structure [36]. The band at 638 cm−1 may be also the effect of vibrations of Fe–O–P bond [36].
Thermal and physical properties
Thermal effects were characterized through assignation of glass transition temperature (Tg) and change of specific heat (Δcp). Relaxation time (τ was calculated accordingly to Eq. 1 [37]. Impact of increase of iron ions was manifested in noted changes of Tg and Δcp values designated from DSC curves (Fig. 2) and in density, molar volume and oxygen packing density. The set of parameters is presented in Table 1.
where β equals to 10° min−1—the heating rate.
Glass transition temperature (Tg) is slightly increasing. This effect is strongly depended on the substitution of ions marked in Fe/(Fe + Ca + Mg) value, and depolymerization degree commonly reflected in O/P ratio.
The general tendency of bands assigned to non-bridging links in various structural phosphate units to downshift, registered particularly in Raman and also in MIR spectra shows that in case of phosphorus-oxygen subnetwork the number of relatively longer and less covalent bonds comparing to bridging bonds in phosphate chain is increasing. Considering the confirmed gradual depolymerization, it can be assumed that progressive shortening of chains leads to massive disruption in the structure. Following [24, 38], it would imply the tendency of Tg to decrease.
Glass transition temperature (Tg) depends on chemical composition and the type of incorporated cations has also a strong impact which cannot be omitted [24]. Progressive substitution of magnesium and calcium oxide with iron oxide leads to the increasing of the number of bonds in which iron ions are involved. The previous work [19] also indicated that simultaneously the number of coordinated both tetra and octahedrally Fe3+ ions is increasing above decreasing of Fe2+ ions in this system.
Thus, a slight increase of Tg value may be rather explained through the differences in cation field strength [38]. The exact values of Z/a2 parameter (Z—valence, a—ionic distance for oxides [Å]) are equal to 0.76 for Fe3+ in octahedral and 0.85 in tetrahedral coordination which is vividly increased compared to the values for magnesium (0.45 in octahedral coordination), calcium (0.33) and even octahedrally coordinated ferrous (0.43) ions [38, 39].
Cations characterized by smaller size and greater charge density such as ferric ions are connected with phosphate anions through oxygen atoms and create bonds stronger than formed by calcium and magnesium ions, and theoretically make the structure more rigid. It also enlarges the required temperature of rotational activation modes [24, 38] and reinforces the structure. It is connected with the increase of Tg value (Fig. 3a), the most significant for 8Fe41P and 15Fe41P glasses. It is also consistent with the statement which confirms that the obtained alteration in Tg value are a consequence of strengthening of the structure [36].
A studied metaphosphate glass 2Fe41P contains more magnesium and calcium ions and simultaneously possesses more P–O–P joints and Mg–O and Ca–O bonds. It changes with the increase of Fe/(Fe + Ca + Mg) parameter which reflects the degree of the substitution of particular ions. Both magnesium and calcium oxygen bonds are less covalent (iG Mg–O = 0.670 and iG Ca–O = 0.707) [40] comparing to Fe2+–O and Fe3+–O (\(i_{{{\text{G}}\,{\text{Fe}}^{2 + } {-}{\text{O}}}} = 0.658\) and \(i_{{{\text{G}}\,{\text{Fe}}^{3 + } {-}{\text{O}}}} = 0.529\)) [40], but these values are still tremendously enlarged in comparison to P–O bond (iG P–O = 0.314) [40]. Therefore, this glass is made of more polymerized units which are based on more covalent P–O–P linkages, modified by significant ionic influence of Mg2+ and Ca2+. Such differentiation causes a certain internal strains in the structure.
It is presumable that the structure of glasses with higher iron oxide addition is reoriented in order to be strengthen and better preserved, thus Fe3+ ions reinforce more depolymerized structure containing significantly shorter chains, dimers and isolated orthophosphates. In all likelihood, iron ions are involved in formation of more ionic and therefore more elastic, however, still adequately strong bonds comparing to P–O–P bridging linkages reduced along with increase of Fe/(Fe + Ca + Mg) ratio.
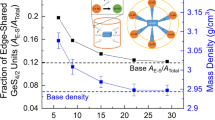
Set of parameters used for the description of physical properties of studied glasses, such as molar volume Vmol and oxygen packing density do calculated from Eqs. 2 and 3 [41], and measured density dr is presented in Table 1. Mentioned parameters increase with gradual iron oxide addition (Fig. 3b-d).
Where xMO is the molar fraction and MMO is the molecular weight of the particular oxide, and dr is experimentally determined density.
Where mo is mass of oxygen atoms in one mole of glass.
The variation of density is due to substitution of ions with smaller atomic weight which are in this case calcium and magnesium ions, by iron ions with higher molecular mass. Such implication makes structure more dense.
Concomitantly, as can be seen in Table 1, the molar volume increases along with Fe/(Fe + Ca + Mg) ratio and reduce of Fe2+/Fetotal ratio. It designates that the structure is more expanded and it correlates with increase of not only the number of non-bridging oxygens [21] but also the number of ferric ions concluded in [19]. Iron redox equilibria impacts the amount of oxygen in the glass. The predominance of ferric ions indicates increase of the number of oxygen atoms [27] which considerably contribute to the volume of the oxygen polyhedral.
Molar volume and oxygen packing density depict the rigidity and compactness in the glass structure [42]. The observed increase of do value implies the higher mass of oxygen atoms per one mole of glass. It can be also concluded that with incorporation of Fe2O3, the structure is more packed. It presumably decreases to a certain point the mobility of components and makes it also slightly more rigid. The certain increase in the rigidity may also explain the minor increase of Tg [43].
Results of the spectroscopic study in glass transition temperature range
Spectra were collected at Tg onset, Tg and Tg endset temperatures designated via DSC method (Table 1) with preservation of constant heating velocity (10° min−1) in order to gain information about structural alterations occurring in glasses containing 2 mol% and 30 mol% Fe2O3. Obtained results were directly connected with glass transition effects revealed in DSC curves (Figs. 4a, 5a). Spectra before heating and after cooling were also recorded and presented in Figs. 4b, 5b.
30Fe41P glass
As it can be seen in Fig. 4a, the collected spectra of 30Fe41P sample at Tg onset, Tg and Tg endset are varied in a subtle way. Spectra obtained from sample before heating and after cooling are in relatively good agreement. Only a slight rise and shifts in bands positions after cooling is observed.
Glass transition effect demarcates the rigid and viscoelastic states. In glass transition temperature, a weak thermal vibrations are induced. They presumably cause the breaking of bonds characterized by higher ionicity. Above Tg, the occurrence of significant change of structurally sensitive properties is also manifested, which is correlated to progressive gradual breaking of oxygen bonds and depolymerization of the network [44].
As it was indicated, iron ions creates less ionic but sufficiently strong bonds, thus the structure exhibits essential resistance. Furthermore, it needs to be noted that based on O/P value, 30Fe41P should be recalled as a glass with high amount of orthophosphate units. Its Raman and MIR spectra collected in room temperature, however, reveal the existence of P–O–P linkages. Corresponding bands assigned to P–O–P linkages located in the 850–950 cm−1 and 700–800 cm−1 regions are only slightly influenced due to temperature treatment (Fig. 4a). Based on that results, it is stated that alteration in polymerization degree at temperatures associated with glass transition effect is elusive.
Certain shifts are, however, visible in Fig. 4b in spectra before heating and after cooling. Changes in location of bands presumably associated with vibrations of bridging oxygens in Q1 units and stretches of (PO4)3− groups indicate that cooling with velocity rate equal to 10 °C min−1 was sufficient to obtain the material containing only slightly more orthophosphate and pyrophosphate units than the starting one. Nevertheless, the detected structural alterations in case of this sample occurring due to glass transition effect are relatively minor.
2Fe41P glass
On the contrary to 30Fe41P glass, in spectra collected from 2Fe41P glass alterations are more visible and it presumably entails more modifications in the structure (Fig. 5a). Observed changes are listed below:
band assigned to asymmetric stretch in (PO2)− groups is becoming to be less distinct.
band in the region 1150–1050 cm−1 is broadening and increasing.
Band located in the region 950–850 cm−1 shifts slightly to higher wavenumbers.
Band in 800–650 cm−1 region exhibits the change in shape
Alterations observed in 950–650 cm−1 region similarly as it was in case of 30Fe41P indicate the increase of the number of shorter chains, pyrophosphate units. Moreover, a decrease of the amount of metaphosphate units may be also concluded from the behavior of band at ~ 1250 cm−1.
Simultaneously, the band at about 1128 cm−1 is rising. In this region, stretching vibrations of non-bridging oxygen links in various phosphate groups are observed. This band should be presumably connected to the band at about 1100 cm−1 observed in spectra obtained in ambient temperature (Fig. 1). The slight change in the area under band may indicate the alterations of the amount of P–O and Si–O bonds and fluctuations of the number of Q2 and Q1 units.
As it was indicated through changes of oxygen packing density, molar volume and glass transition temperature, structure of 2Fe41P in its parent state is less dense and packed, contains more ionic Ca–O and Mg–O bonds and is characterized by more polymerized network. It is presumable that it possesses more flexibility. Nevertheless, value of obtained relaxation time for 2Fe41P glass is higher than for 30Fe41P glass.
As can be seen in Fig. 5b, spectra before heating and after cooling differ considerably. The states obtained after cooling (Fig. 5b) and observed within the glass transition effect presented in Fig. 5a are found to be relatively similar. It implies a good preservation of the structure received in glass transition temperatures ranges. It also indicates that in contradiction to 30Fe41P glass, alterations in 2Fe41P glass spectra are far more progressive.
Conclusions
Results obtained in MIR study at room temperature confirmed that materials with lower iron oxide addition are more polymerized. This statement was concluded through deliberations about the changes in the 1300–1050 cm−1 and 950–650 cm−1 regions corresponded with vibrations of non-bridging oxygens and stretches of P–O–P linkages.
Alterations in thermal properties detected in glasses from P2O5–SiO2–K2O–MgO–CaO–Fe2O3 system are considerably minor. A slight augmentation of Tg and Δcp values with incorporation of Fe2O3 is explained by greater field strength of ferric ions. Glass transition effect is influenced by many factors and exhibits high complexity. Present work states that the degree of polymerization and the relations between glass components concluded from bonds ionicity needs to be also taken in considerations.
Displayed tendency of physical properties confirmed the impact of variations of chemical composition on the structure of studied materials. Glasses with higher iron oxide addition are more dense and packed. However, it does not entail the decrease in molar volume, what marks the expansion of the structure.
Designated value of time relaxation (τ) occurred to be higher for more polymerized 2Fe41P glass. It was concluded from DRIFT spectra collected in temperature ranges attributed with glass transition effect that the alterations in the structure are more significant in this sample in comparison to detected modifications in 30Fe41P. The response to thermal treatment was also pronounced and revealed through comparison of spectra obtained before heating and after cooling. 2Fe41P sample is found to be less resistant than 30Fe41P glass.
Due to smaller alterations in 30Fe41P glass revealed via diffused reflectance Fourier transform infrared spectroscopy, it may be assumed that iron oxide addition makes the glass more resistant in designated temperature range. It is presumably also associated with the reduced number of more ionic Ca–O and Mg–O bonds.
The 30Fe41P sample is more resistant to thermal treatment in designated temperature range. It is presumable that the ferric ions incorporation leads to the reinforcement of the structure of iron pyrophosphate glass from P2O5–SiO2–K2O–MgO–CaO–Fe2O3 system.
References
Sales BC. Phosphate glasses. Mater Res Soc. 1987;12:32–4.
Yin Q, Kang S, Wang X, Li S, He D, Hu L. Effect of PbO on the spectral and thermo-optical properties of Nd3+-doped phosphate laser glass. Opt Mater. 2017;66:23–8.
Pisarski WA, Żur L, Goryczka T, Sołtys M, Pisarska J. Structure and spectroscopy of rare earth—doped lead phosphate glasses. J Alloys Compd. 2014;587:90–8.
Podniesiński D, Nakielska M, Kozłowska A, Stępień R, Pysz D. Laser na szkle fosforanowym domieszkowanym erbem, iterbem i chromem. Electron Mater. 2015;43(1):4–10.
Hu L, He D, Chen H, Wang X, Meng T, Wen L, Hu J, Xu Y, Li S, Chen Y, Chen W, Chen S, Tang J, Wang B. Research and development of neodymium phosphate laser glass for high power laser application. Opt Mater. 2017;63:213–20.
Stoch P, Stoch A, Ciecińska M, Krakowiak I, Sitarz M. Structure of phosphate and iron-phosphate glasses by DFT calculations and FTIR/Raman spectroscopy. J Non-Cryst Solids. 2016;450:48–60.
Ojovan MI, Lee WE. Immobilisation of radioactive waste in glass. In: Ojovan MI, Lee WE, editors. An introduction to nuclear waste immobilisation. 2nd ed. Amsterdam: Elsevier; 2014. p. 245–82.
Wacławska I, Szumera M. Fosforanowe materiały ceramiczne do immobilizacji kadmu w środowisku glebowym. Ceram Mater. 2011;63(2):407–12.
Abou Neel EA, Ahmed I, Pratten J, Nazhat SN, Knowles JC. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials. 2005;26:2247–54.
Ahmed I, Shaharuddin SS, Sharmin N, Furniss D, Rudd C. Core/clad phosphate glass fibres containing iron and/or titanium. Biomed Glasses. 2015;1:20–30.
Mishra A, Rocherullé J, Massera J. Ag-doped phosphate bioactive glasses: thermal, structural and in vitro dissolution properties. Biomed Glasses. 2016;2:38–48.
Yang JH, Park H-S, Cho Y-Z. Silver phosphate glasses for immobilization of radioactive iodine. Ann Nucl Energy. 2017;110:208–14.
Brow RK. Review: the structure of simple phosphate glasses. J Non-Cryst Solids. 2000;263&264:1–28.
Bingham PA, Hand RJ, Forder SD. Doping of iron phosphate glasses with Al2O3, SiO2 or B2O3 for improved thermal stability. Mater Res Bull. 2006;41:1622–30.
Liebau F. The influence of cation properties on the conformation of silicate and phosphate anions. In: Navrotsky A, O’Keeffe M, editors. Structure and bonding in crystals. London: Academic Press, Inc; 1981. p. 198.
Moustafa YM. Characterization of iron oxychloride potassium phosphate glasses. J Phys D Appl Phys. 1999;32:2278–86.
Li H, Wang C, Yu H, Liang X, Yang S. Effects of calcium oxide addition on the structure and thermal properties of iron phosphate glasses. Spectrosc Lett Int J Rapid Commun. 2015;48(3):184–9.
Mošner P, Račický A, Koudelka L. Thermal properties and crystallization of MgO–FeOx–P2O5 glasses. J Therm Anal Calorim. 2018;132(2):843–50.
Kuczek J, Jelen P, Stoch P, Błachowski A, Wacławska I, Szumera M. Raman and Mossbauer studies of iron phosphate-silicate glasses. J Mol Struct. 2018;1170:82–9.
Liang X, Yin G, Yang S, Lai Y, Wang J. Lanthanum oxide effects on the structure of calcium phosphate glasses. Spectrosc Lett. 2011;44:418–23.
Li HJ, Liang XF, Yu HJ, Yang DQ, Yang SY. Studies of structure of calcium–iron phosphate glasses by infrared, Raman and UV–Vis spectroscopies. Indian J Phys. 2016;90(6):693–8.
Shaim A, Et-tabirou M. Role of titanium in sodium titanophosphate glasses and a model of structural units. Mater Chem Phys. 2003;80:63–7.
Szumera M, Wacławska I, Sułowska J. Influence of CuO and ZnO addition on the multicomponent phosphate glasses: spectroscopic studies. J Mol Struct. 2016;1114:78–83.
Hudgens JJ, Martin SW. Glass transition and infrared spectra of low-alkali, anhydrous lithium phosphate glasses. J Am Ceram Soc. 1993;76(7):1691–6.
ElBatal FH, Hamdy YM, Marzouk SY. UV–visible and infrared absorption spectra of transition metals-doped lead phosphate glasses and the effect of gamma irradiation. J Non-Cryst Solids. 2009;355:2439–47.
Qian B, Liang X, Yang S, He S, Gao L. Effects of lanthanum addition on the structure and properties of iron phosphate glasses. J Mol Struct. 2012;1027:31–5.
Fang X, Ray CS, Mogus-Milankovic A, Day DE. Iron redox equilibrium, structure and properties of iron phosphate glasses. J Non-Cryst Solids. 2001;283:162–72.
Dube CL, Stennett MC, Gandy AS, Hyatt NC. Simulation of alpha decay of actinides in iron phosphate glasses by ion irradiation. Nucl Instrum Methods Phys Res B. 2016;371:424–8.
Corbridge DEC, Lowe EJ. The infra-red spectra of some inorganic phosphorus compounds. J Chem Soc. 1954;1:493–502.
Majjane A, Chahine A, Et-tabirou M, Echchahed B, Do T-O, Mc Breen P. X-ray photoelectron spectroscopy (XPS) and FTIR studies of vanadium barium phosphate glasses. Mater Chem Phys. 2014;143:779–87.
Efimov AM. IR fundamental spectra and structure of pyrophosphate glasses along the 2ZnO P2O5–2Me2O P2O5 join (Me being Na and Li). J Non-Cryst Solids. 1997;209:209–26.
Doweidar H, Moustafa YM, El-Egili K, Abbas I. Infrared spectra of Fe2O3–PbO–P2O5 glasses. Vib Spectrosc. 2005;37:91–6.
Gabelica-Robert M, Tarte P. Infrared spectrum of crystalline and glassy pyrophosphates: preservation of the pyrophosphate group in the glassy structure. J Mol Struct. 1982;79:251–4.
Mandal S, Hazra S, Ghosh A. Glass formation in the PbO–Fe2O3 system with high PbO content. J Mater Sci Lett. 1994;13:1054–5.
Moustafa YM, El-Egili K, Doweidar H, Abbas I. Structure and electric conduction of Fe2O3–P2O5 glasses. Phys B. 2004;353:82–91.
Lu M, Wang F, Liao Q, Chen K, Qin J, Pan S. FTIR spectra and thermal properties of TiO2-doped iron phosphate glasses. J Mol Struct. 2015;1081:187–92.
Sułowska J, Wacławska I, Szumera M. Effect of copper addition on glass transition of silicate-phosphate glasses. J Therm Anal Calorim. 2012;109:705–10.
Ma L, Brow RK, Ghussn L, Schlesinger ME. Thermal stability of Na2O–FeO–Fe2O3–P2O5 glasses. J Non-Cryst Solids. 2015;409:131–8.
Varshneya AK. Fundamentals of inorganic glasses. London: Academic Press Inc; 1993. p. 35.
Görlich E. The effective nuclear charges and the electronegativity. Kraków: Polish Academy of Art and Science; 1997.
Fredholm YC, Karpukhina N, Law RV, Hill RG. Strontium containing bioactive glasses: glass structure and physical properties. J Non-Cryst Solids. 2010;356:2546–51.
El-Mallawany R. Tellurite glass smart materials: applications in optics and beyond. Berlin: Springer; 2018.
El-Mallawany R. Thermal properties and crosslinking of binary TeO2–Nb2O5 and TeO2–WO3 glasses. J Non-Cryst Solids. 2013;379:177–9.
Stoch L. Structure and crystallization of multicomponent glasses. In: International Congress on Glass, vol. 1 (2001), p. 62–73.
Acknowledgements
The work was supported by the Faculty of Materials Science and Ceramics AGH—University of Science and Technology No. 11.11.160.617 and the EU Project POWR.03.02.00-00-I004/16.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kuczek, J., Jeleń, P., Sułowska, J. et al. Correlation between glass transition effect and structural changes in multicomponent iron phosphate-silicate glasses. J Therm Anal Calorim 138, 4145–4153 (2019). https://doi.org/10.1007/s10973-019-08465-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08465-5