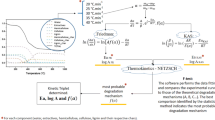
Abstract
Motivated by green building applications (bio-composite and insulation materials), thermogravimetric and kinetic analysis is applied to investigate the thermal degradation of cleaned hemp fibers (F), obtained after water retting and mechanical decortication of dioecious plant stalks, and technical fiber (TF), obtained after field retting of monoecious plant stalks. Celluloses (microcrystalline PH105 cellulose and cotton linter cellulose) are used for comparison. F and TF dynamic curves are well described by a four-step scheme. The dominant one concerns pseudo-cellulose decomposition with the release of 70 and 54 mass% volatile matter, respectively. The corresponding activation energies are in the range of typical cellulose values (223 and 211 KJ mol−1). Fiber pretreatments (water washing, mild torrefaction, mercerization) modify the pseudo-cellulose content (release of 73–80 mass% volatile matter) and properties, as testified by the higher activation energies (229–248 kJ mol−1) of the decomposition process. The decortication method also contributes remarkably to the characteristics of the fiber cellulose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Significant scientific and industrial interest is currently focused on green building materials, such as insulating materials and fiber-reinforced composites, which exploit natural fibers [1]. These are cellulosic fibers embedded in a matrix essentially consisting of pectin, hemicellulose and lignin, exhibiting mechanical properties comparable with those of synthetic fibers. The stalks of industrial hemp (Cannabis sativa L.) are constituted by two main concentric layers, an internal woody core and an outer bark which contains long bast fibers. This is a valuable fiber owing to high cellulose contents (typically around 65–78 mass% with 12–17 mass% hemicellulose and 2–7 mass% lignin) and high strength (300–1800 MPa) [2]. The cellulose contents generally increase with the growing stage, and, at the flowering stage, hemicellulose starts to decrease to the advantage of lignin [3]. Chemico-physical properties of the fibers, apart from the influences of cultivar, geographical origin, harvesting period and season variability, are highly affected by the extraction method, including retting and separation from the stalks, that can be difficult and costly [4].
Typically retting is a biological process apt to separate the bast cellulosic fibers from the other stalk macro-components. Removal of non-cellulosic materials, such as pectin, hemicellulose and lipophilic extractives, and inorganics indirectly, causes an increase in the percentage of cellulose in the fibers [5, 6]. Galacturonic acid and rhamnose (pectin) and xylose (hemicellulose) are metabolized with reduction factors around 35, 20 and 50%, in the order [5]. Conversely, the lignin fraction becomes more concentrated with values up to around 7 mass%, versus 5 mass% of un-retted samples, though changes in the chemical composition may also modify the accuracy of the Klason method of lignin quantification [6].
Following retting, the crystalline index of cellulose increases from about 53 (un-retted samples) up to 73% [6]. Amorphous components between the cellulose micro-fibrils in un-retted fibers give rise to disoriented zones and, in this way, affect the crystallinity characteristics and the tissular cohesion of cellulose. Other mechanical pretreatments, such as decortication and carding, can also affect the chemico-physical properties of the fiber [4], though the cellulose crystallinity seems to remain unmodified.
Undoubted advantages of hemp fibers are associated with renewability, sustainability, low cost, low density, no impact on global warming, biodegradability and non-abrasive properties during processing [4, 7]. These advantages are also pertinent for the new bio-based insulation materials which, for hemp, include concrete inside a wood frame structure, natural wool for insulation, or hemp-lime mortar for coating [1]. However, as typical of lignocellulosic materials [8], the thermal stability is low, thus leading to high flammability which is a serious drawback for hemp product applications in the sector of green materials. This aspect is also of importance during bio-composite manufacturing owing to the deterioration of mechanical properties in consequence of thermal stresses [9].
The thermal decomposition of hemp stalks and shives has been recently examined in the view of bioenergy and bio-fuel productions (for instance, among the most recent works see [10,11,12,13,14]). Moreover, in some cases, investigations also consider the flammability of non-woven fibers [15] or are focused on the search of a relation between the fiber chemical composition and their properties (thermal stability and/or mechanical strength) and the effects of pretreatments [7, 9, 16,17,18,19]. A high cellulose content is generally considered a favorable condition for improving thermal stability and structural properties, features that make the fibers suitable for reinforcement in bio-composites. Solvent extraction, mercerization and enzyme scouring [17] increase the cellulose purity and structural ordering, which are also required for obtaining a good fiber matrix adhesion [20], though severe treatment conditions may also cause structural disruption [21].
Despite the remarkable knowledge of the hemp fiber properties, the understanding of the degradation kinetics is lacking, as only highly simplified analyses are currently available. Iso-conversional methods [22], applied to thermogravimetric curves of hemp fibers, show that the decomposition process cannot be described by a single-step reaction [17] (conversions between 15 and 65% require activation energies of 130–190 kJ mol−1) and that at least a low- and a high-conversion mechanism is required [7] (activation energies in the range 150–190 kJ mol−1 and 190–205 kJ mol−1, respectively). However, iso-conversional methods are generally unsuitable to analyze multi-step kinetics [23]. Hence, due to complex and overlapping decomposition of fiber components, accurate kinetic models have not yet been developed, capable of describing the evolution of the fiber components over the entire temperature range of interest and the impact of pretreatments.
The aim of this study is to model the decomposition kinetics of hemp fiber for the entire range of decomposition temperatures, using cellulose samples for comparison, and how it is affected by several pretreatments (manual decortication, hot water washing, mild torrefaction, mercerization). Dynamic thermogravimetric curves are measured, at different heating rates, of the various samples and are interpreted by means of a multi-step scheme, leading to parameter estimation for the intrinsic decomposition kinetics.
Materials and methods
In this section, the samples and pretreatments are first described. Then, the conditions of the thermogravimetric measurements and the results of the proximate analysis are presented.
Fiber samples
The fibers examined in this study, as made available from the producer, are originated from the variety of Cannabis sativa Eletta Campana, grown in Sud Italy (Capodrise, CE) which was already considered in previous studies of this group [11, 14]. The bast fibers (F) are obtained from the stalks of dioecious plants (height around 3 m, with stalks harvested from 0.2 to 2 m height), specifically grown for fiber production. After water retting and drying, mechanical decortication is applied, followed by cleaning, motivated by applications for textile and high-added value products. The so-called technical fiber (TF) is obtained from monoecious plants (height around 1.5 m with stalks harvested from 0.05 to 0.5 m), grown for seed production. After field retting, for about thirty days, which allows for the separation of the large part of the shives, fibers are cut into pieces 4–5 cm long. This is a by-product exploited as thermal and acoustic insulating material in the green building sector.
Cellulose samples
Given the major share of cellulose in the chemical composition of the hemp fibers and the remarkable number of studies about decomposition kinetics, cellulose samples are also considered. These include microcrystalline cellulose Avicel PH 105 (Vivapur, JRS Pharma GMB), indicated in the following as avicel, and cotton linter cellulose (Fluka cotton linters, CAS number 9004-34-6), indicated in the following as CL. The former is a purified partially depolymerized α-cellulose having average particle sizes of 15 micron. Cotton linter cellulose (α-cellulose) is an acid-washed powder with fiber lengths between 0.02 and 0.15 mm.
Fiber pretreatments
The conditions of water retting as well as the successive decortication may have an important impact on the properties of both fibers and shives [4, 11]. So, to evaluate the effects of mechanical decortication, fibers manually separated in laboratory, indicated with the acronym F*, are examined, as obtained from the stalks subjected to water retting and drying under the same conditions as those of the F samples.
Other F pretreatments include hot water washing (WF), mild torrefaction (TORF) and mercerization (MF) applied to 5 mm long pre-dried particles. The pretreatment conditions are coincident with those already reported by other researchers displaying effectiveness without damaging the cellulose structure. More precisely, the hot water pretreatment is carried out for a sample to distilled water ratio of 100 g L−1 with a temperature of 353 K and a washing time of 2 h under continuous stirring [24, 25]. This is a widely used pretreatment to remove/to reduce the alkali contents in lignocellulosic fuels together with some organic matter, in particular water-soluble components [26] and other compounds possibly generated from extremely mild hydrolysis. Very mild torrefaction, which remove the low-temperature components without damaging the cellulosic fibers [19], is accomplished with a heating rate of 5 K min−1 up to 573 K with zero holding time (10 mg sample pyrolyzed by means of the thermogravimetric analyzer described below). The process leads to a reduction in the sample mass by 15%. It is worth noticing that torrefaction for final temperatures of 423 K and 523 K does not appear to cause significant effects on the subsequent decomposition process (not considered in the following). Mercerization [17] is made with an 8 wt/v % concentration of NaOH solution (2.5 g/100 mL) for 1 h at 303 K. The sample is then subjected to washing with running tap water, followed by distilled water, for eliminating alkali from the wash water.
Thermogravimetric analysis
Thermal decomposition of pulverized samples (avicel and CL celluloses, F, TF, F*, WF, TORF and MF), with initial mass equal to 2 mg, is investigated using the commercial system Mettler TGA/1. The inert environment is created by means a nitrogen flow of 50 mL min−1. Samples are heated up to 773 K with rates of 5, 10 and 20 K min−1. All samples are subjected to oven drying overnight at 353 K after preliminary crushing and grinding. For the fibers, it is difficult to precisely determine the particle size distribution, owing to aggregation phenomena and formation of wads, during the sieving process, as can be observed from the snapshot of Fig. 1 A. Hence, the basic information on the particle sizes is gained by means of SEM images of the samples (not shown) which reveal that the minimum and maximum diameters are around 10 and 75 micron, respectively. The length of the fibers is around 1–2 mm.
Thermogravimetric measurements are carried out in triplicate, showing a very good repeatability. Given that the main objective of the study is the kinetic analysis, it is important to guarantee conditions of chemical kinetics control. Hence, particular care has been put in selecting a low initial sample mass that, for the moderate heating conditions of the experiments, allows for negligible interferences of heat and mass transfer phenomena. As already reported in a previous study [14], further reduction in the initial sample mass does not introduce any change in the measured data, thus confirming kinetic control. Moreover, yields of charred residue independent from the initial sample mass also indicate that the activity of secondary reactions is negligible.
Proximate analysis
Samples are characterized in terms of proximate analysis. Thermogravimetry is applied for such a purpose, following the procedure already described elsewhere [25]. Results of the proximate analysis are reported in Table 1. The highest quantity of inorganics is observed for the TF sample owing to field retting, and possible soil contamination, and the lack of any kind of decortication. The proximate analysis of the F sample is quantitatively close to that of the CL cellulose (the slightly higher ash of the F fibers is associated with a corresponding lower FC, that is, 5 versus 6 mass%). Avicel and CL cellulose own the lowest ash content and, conversely, the highest VM value (96 mass%). As already found for shives [14], manual decortication is less effective for ash removal (ash content of 1.6 vs. 0.6 mass% for the F* and F samples, respectively).
The FC content of the WF and MF samples is higher than that of the untreated fiber F. This is a somewhat unexpected result as the hemicellulose/pectin and ash contents are expected to be reduced. However, these pretreatments cause significant fiber swelling and agglomeration (see the snapshot reported in Fig. 1B) which, combined with the relatively high mass and mainly large volume of the sample inside the crucible for the proximate analysis tests, highly enhance the activity of secondary reactions owing to a prolonged contact between primary vapors and nascent char. Consequently, secondary char, whose formation is especially important for cellulose vapors [27], is likely to produce a significant contribution in the FC contents leading to values higher than expected (FC values of 7–10 mass% versus 5 mass% of the untreated F sample). It is also useful observing that, at the conclusion of the tests for the pretreated samples, the crucible walls become black owing to deposition of polymerized vapors, a feature that is not observed in the other cases. Finally, the higher FC content of the TORF sample is a well-known result of the torrefaction process [28], in consequence of the partial devolatilization.
Results
In this section, the thermogravimetric behavior is first described of the F and TF samples including the effects of the pretreatments and the comparison with celluloses. Then, the results of the kinetic modeling are presented and discussed.
Thermogravimetric characteristics
The thermogravimetric analysis uses the classical parameters previously introduced [11, 14, 25], such as the initial degradation temperature corresponding to a mass fraction of 0.98, Tinitial, the temperature Tpeak of the maximum devolatilization rate, with the corresponding − (dY/dt)peak and Ypeak, Toffset which demarcates the beginning of the final region of the rate curve, the char yield, Y773 (the solid mass fraction detected at 773 K), and the full width of the rate curve at half maximum, FWHM (temperature range). The integral and differential curves for the samples F, TF, F*, Avicel and CL are reported in Fig. 2 A (heating rate 5 K/min). As decortication is a preliminary operation needed for fiber separation from the shives, the behavior of the sample F* (manual decortication) is not associated with the post-treatments of the F sample motivated by the need of property improvement and examined in the following.
The qualitative feature common to all curves is a clearly defined high peak rate. The position, as well as its value, however, are sample dependent. As a first remark, it can be noticed that the avicel sample peaks about 20 K before the CL cellulose with a slightly higher value (about 20% higher). Another difference, between the two samples, is that the temperature range FWHM is narrower for the avicel (31 vs. 36 K). In summary, the avicel cellulose decomposes with a well-defined peak rate over a narrower range of lower temperatures. Instead, an initial zone of low decomposition rates is shown by the CL cellulose together with a tailing zone. On the other hand, specific behaviors are reported [29] for various celluloses, for instance differences up to 30 K for the position of the peak rate between avicel cellulose and Whatman paper. Following the interesting and thorough analysis reported in [30], compared with CL, avicel cellulose owns a lower purity in glucose content (about 4 mass% of xylan/mannan), a wider molar mass distribution and a lower average degree of polymerization, properties that shift the degradation peak at lower temperatures (the impact on the decomposition process is high for the first two properties and intermediate for the last one). Moreover, amorphous portions of the cellulose structure, if partially preserved, exhibit lower thermal stability and start to degrade at lower temperatures [7, 29]. Also, the degradation temperature of crystalline cellulose increases with the crystallite size [31]. It is worth noticing that crystalline and amorphous regions are found in cotton linters, in proportions depending on the genotype, geographical origin and pretreatments for cellulose pulping [32], as well in microcrystalline cellulose [33]. Hence, the different properties of the two celluloses result into remarkably different thermogravimetric characteristics.
As well known, the absolute peak of the rate of lignocellulosic material decomposition in thermogravimetry is mainly due to the cellulose macro-component [11, 14, 25]. At a first glance, the F and TF samples peak at about the same temperature (621 and 617 K) though the former peak is about 42% higher. These findings are justified by the higher amounts of non-cellulosic components, and most likely of inorganics, of the TF sample. Consequently, for this sample, the shoulder and tail zones are more important. As already observed [11, 14], the characteristics of the decortication process have important implications on the properties of both fibers and shives. It is confirmed that manual separation is far less effective for the eliminations of non-cellulosic components from the fibers (the peak rate is anticipated by 13 K and reduced by about 18%, the FWHM is wider by 8 K). Incidentally, the F* peak rate is slightly higher than that of the TF sample, presumably owing to the lower ash content, but localized at lower temperature, most likely in consequence of the still significant contents of shives (the origin plants are, however, different).
It is interesting noticing that, from the quantitative point of view, the F sample shows the same position (621 K) and a value only slightly lower (about 6%) of the peak rate with respect to the CL cellulose. A significant difference between the two samples is that FWHM is narrower for the F sample. It can be speculated that, apart from the slightly lower content, the in situ cellulose of the F fiber peaks as the CL cellulose though over a slightly narrower temperature range, indicating quantitatively close properties. It is reported [7] that, compared with other fibers such as jute, flax, hemp and kenaf, cotton fibers exhibit higher cellulose contents which, however, are characterized by higher amounts of amorphous portions. This may partly explain the small differences between the two samples.
The thermogravimetric curves for the samples WF, MF and TORF are compared with those of the F sample in Fig. 2B. Though the chief characteristics are not modified by the pretreatments, important differences can be observed between the TORF sample and the other two. Mild torrefaction appears to have a small effect on the peak rate, which is barely anticipated, and the FWHM range, though the devolatilization rates are higher than those of the untreated fibers for the temperatures around 590–620 K. This result may be due to an increase in the relative percentages of both cellulose and inorganic matter following mild torrefaction. Indeed, the volatile matter is partly released from the more thermally labile non-cellulosic components, while the solid residue remains in the same position as in the original material, also leading to higher char yields. It can be speculated that the increase in the cellulose content is not associated with an improvement in its properties, in particular the crystallinity [19].
The position of the peak rate remains practically unvaried following the washing and mercerization pretreatments. However, the FWHM range is significantly reduced (25 vs. 30 K) and the peak rate increases (by 18–29%), especially for the mercerization case. Moreover, in contrast with the results of proximate analysis, given a kinetic control and a negligible activity of secondary reactions in the conversion processes, both pretreatments lead to lower char yields.
The values of peak rate and corresponding positions, as well those of other characteristic temperatures, rates and mass fractions are reported in Table 2 for all the samples, as the heating rate is varied. The features above described are preserved though, as already known, the peak rate tends to increase and to be reached at successively higher temperatures. In practice, the narrow range of heating rates (5–20 K min−1) gives rise to relatively small variations in the measured curves. Variations on the temperatures of the peak rate are between 3–4% (temperature differences between 18 and 25 K). Differences are also small on the FWHM ranges with typical differences around 5 K. Instead, more important are the variations on the peak rates with factors of increase between 2.5 and 2.7.
Kinetic modeling
The kinetic modeling of the thermogravimetric curves uses the well-established approach based on the assumption that lumped classes of volatiles are released by a set of parallel reactions referred to the sample macro-components (for instance [14, 34,35,36,37,38,39]) though the rate laws and other model features can be slightly different among the various formulations [40]. The overall mass loss rate is then the simple summations of the single rates. The number of reactions steps (or components) generally is a parameter to be estimated. However, preliminary evaluations made for all the samples (F, TF, F*, WF, MF, TORF), using the findings reported above and the understanding gained in the kinetic modeling of the hemp stalk decomposition [14], shows that four steps are sufficient for accurate predictions:

where S is the sample which produces the lumped volatile products Vi (i = 1–4). To be precise, volatiles are referred to the pseudo-components. Indeed, a rigorous separation is difficult of the contributions of the macro-components or their parts while carrying out the kinetic analysis of the mass loss curves. The reactions rates show an Arrhenius dependence (Ai are the pre-exponential factors and Ei the activation energies) on the temperature and a linear dependence on the mass fractions of lumped volatile product classes released. The selection of adequate operating conditions allows the sample temperature to be assumed coincident with the heating temperature (T = T0 + ht, where T0 is the initial temperature, h is the heating rate and t is the time). Then, the mathematical model consists of n ordinary differential equations for the mass fractions, Yi, of the lumped volatile products:
where νi (stoichiometric coefficients) are the initial values.
The kinetic parameters are estimated through the numerical solution (routine ode15s of the software MATLAB) of the mass conservation Eqs. 1 and the application of a method based on the simultaneous nonlinear regression of the curves for the various heating rates for the minimization of the objective function (routine fminsearch), using both mass fractions and time derivatives of the mass fractions [14, 34,35,36, 41]. The parameters to be estimated are the activation energies (E1–E4), the pre-exponential factors (A1–A4) and the stoichiometric coefficients (ν1–ν4), for each sample. Deviations between measurements and model predictions, devTG and devDTG for the integral and differential data, respectively, are defined as in [41].
As already stated, a preliminary evaluation has been carried out for the various fiber samples to determine the minimum number of steps allowing for acceptable predictions. The first stage has examined the possibility to apply the five-step scheme previously developed [14] for the raw, water-retted and hot-water washed hemp stalks, using the corresponding kinetic parameters as the starting values. Most likely, in consequence of the reduced contents of lignin of the fiber samples, with respect to the origin hemp stalk, it has been found that the tail zone (pseudo-lignin decomposition) does not require two-steps. Hence, the steps 1–2 describe the decomposition of low-temperature pseudo-components (pectin and hemicellulose), the step 3 refers to pseudo-cellulose and finally the step 4 to pseudo-lignin. The kinetic evaluation is carried out by requiring the same activation energies for the pseudo-hemicellulose and pseudo-lignin decomposition as common parameters are often assumed for samples showing similar features [42]. Hence, the chief differences among fiber samples are taken into account by a variable activation energy for the degradation of the major component, pseudo-cellulose (in addition to pre-exponential factors).
The thermal decomposition of avicel cellulose is generally described by a single step with high activation energy [29]. On the other hand, as discussed above, the shape of the decomposition curve for the CL cellulose is significantly different. Hence, a preliminary evaluation about the number of steps to be used for cellulose decomposition has again been carried out. It can be stated that, for the avicel sample, a single step is effectively adequate. Instead, for the CL cellulose, in addition to the central major contribution, the introduction of three additional steps (in number of two for the initial zone and one for the final one) significantly improves the quality of the predictions. To match these features, for the CL sample, both a single-step (similarly to avicel) or a four-step (similarly to fibers) scheme is evaluated.
Kinetic analysis
The curves of the cellulose samples are evaluated assuming, as a first part of the analysis, a single step and an initial value of the activation energy around 200 kJ mol−1, based on previous literature results [29]. The results of the optimization process, in terms of kinetic parameters, are listed in Tables 3 A, B. A difference between the activation energy of the avicel and the CL samples can be observed (215 vs. 203 kJ mol−1) which, as already discussed [29], can be attributed to the different chemical properties of the samples. The visual observation of the predicted and measured global mass loss curves at the various heating rates (Fig. 3 A, B) as well as the deviations (Table 3 A) confirm the effectiveness of the approach for the avicel cellulose, whereas the results for the CL cellulose are less satisfactory. For this, the application of a four-step scheme significantly improves the quality of the predictions (Fig. 3 B). The inclusion of two steps for the low-temperature zone and one step for the high-temperature zone leads to a relatively small reduction in the volatile released from the central zone (84 vs. 92 mass%) and, at the same time, an increase in the related activation energy (Tables 3 A, B). This becomes nearly the same as that for the Avicel cellulose (212 vs. 215 kJ mol−1). Hence, the differences in the cellulose properties can also be described by a modified kinetic scheme (as noticed above, for the same scheme consisting of a single step, different activation energies are required).
The results obtained for the kinetic parameters of the four-step scheme for all the fiber samples are listed in Tables 3 A, B. A comparison between predictions and measurements for the global mass loss curves at various heating rates can be made through Figs. 4A, C (F, TF and F*) and Figs. 5A, C (TORF, WF and MF). The deviations reported in Table 3 A and the plots show that the agreement between predictions and measurements is always very good. It is interesting observing that the pseudo-cellulose activation energy for the F, F* and TF samples varies over the narrow range of values 211–223 kJ mol−1, comparable to the value of 212 kJ mol−1 for the dominant component of the four-step scheme applied for the CL cellulose. The activation energies of the two low-temperature steps, which are also the same for the four-step scheme of CL cellulose, are very close (119 and 160 kJ mol−1) to those already estimated for the hemp stalk decomposition [14]. Instead, for the tail zone (pseudo-lignin decomposition), the activation energy, around 126 kJ mol−1, is the same as that of the second more global step evaluated for this component in the five-step mechanism. So, it can be reasonably thought that this step incorporates different contributions, characterized by slow devolatilization rates, which are difficult to separate. The main differences in the decomposition process, following the pretreatments, is again summarized by the activation energy of the pseudo-cellulose with values of 229 kJ mol−1 (TORF), 244 kJ mol−1 (WF) and 248 kJ mol−1 (MF) (vs. 223 kJ mol−1 for the F sample). Hence, the increase in the activation energy of pseudo-cellulose decomposition is generally quantitatively more marked for the pretreatments with respect to the TF and F* samples (211 and 213 kJ mol−1, respectively).
Information on the component dynamics can be gained from Figs. 6, 7 (comparison between the components from F and TF samples, and F and MF samples, respectively) for a heating rate of 5 K min−1 and the stoichiometric coefficients reported in Table 3 B. Moreover, the curves of the volatile released from the pseudo-cellulose components are reported in Fig. 8 (CL, F, TF and F* samples) and Fig. 9 (F, MF, TORF and WF samples) again for a heating rate of 5 K min−1 (the avicel curve is not included given the large differences in the behavior of this sample and those of the other cellulosic components). For the first group of samples, the pseudo-cellulose decomposition releases volatiles corresponding to 70 mass% (F), 61 mass% (F*) or 54 mass% (TF), versus 84 mass% of the CL cellulose described with the four-step scheme (Table 3 B). Then, it is from the second step (pseudo-hemicellulose decomposition) that significant volatile matter is released (7, 11 and 13 mass%, respectively). The amounts of volatile matter released from the pseudo-cellulose of the TORF, WF and MF samples are 73, 77 and 80 mass%, in the order, versus 70 mass% for F sample (Table 3 B). Conversely the amounts of volatile released for the step 2 decrease to 3–5 mass% (vs. 7 mass% of the F sample).
A, B Mass fraction, Y, and mass loss rate, − dY/dt, for A hemp fiber (F), hemp technical fibers (TF), hemp fiber obtained by means of manual decortication (F*), avicel cellulose (avicel) and cotton linter cellulose (CL), and B hemp fiber (F) and hemp fiber after mild torrefaction (TORF), hot water washing (WF) and mercerization (MF), versus temperature (heating rate 5 K min−1, up to 773 K)
A, B Comparison between predictions (data in Table 3 A, B) (lines) and measurements (symbols) of mass fraction, Y, and mass loss rate, − dY/dt, for A avicel and B CL celluloses versus temperature (heating rates 5, 10 20 K min−1, up to 773 K). A one-step mechanism is applied for avicel, both a one-step (dashed lines) and a four-step (solid lines) mechanism is applied for CL
Predicted mass loss rate (solid lines, data in Table 3 A, B), − dY/dt, from the component n.3 (pseudo-cellulose) of the hemp fibers (F), the hemp technical fiber (TF) and the hemp fiber obtained by means of manual decortication (F*), and the component n.3 of CL cellulose, versus temperature (heating rate 5 K min−1, up to 773 K)
The stoichiometric coefficients for the steps n.1 and n.4 weakly vary with the heating rate to take into account the decrease in the char yields as the average reaction temperature increases (average values can be used without significant loss in the accuracy). The component 1 comprises significant amounts of volatile matter only for the TF and F* samples (average values of about 7.5 and 6.5 mass%) in consequence of the absence of a decortication process or a noninvasive (manual) one. The volatile matter evolved from component 1 is lower for the sample F subjected to mechanical decortication (3 mass%) and is further reduced by the pretreatments (1–2 mass%). Furthermore, again with the exceptions of the TF and F* samples, with values corresponding to 6.5 and 7.5 mass%, respectively, the amounts of volatiles released in the tailing zone (pseudo-lignin) are around 5–6 mass% and remains roughly unvaried following the pretreatments.
It is interesting observing that the activation energy for the pseudo-cellulose decomposition increases with the corresponding amount of volatile released (and the corresponding peak rate). It is also evident that the lower is the FWHM of the rate of volatile production from pseudo-cellulose, the higher the activation energy, similarly to the behavior observed for char oxidation [43]. Hence, the method of fiber production and pretreatments remarkably modifies not only the cellulose content (from 54 mass% of the technical fibers TF to 80 mass% of the mercerized fibers MF) but also the characteristics of the decomposition process and thus, the cellulose properties, namely the crystallinity index. Indeed, a correlation is reported [44] between this parameter and the activation energy, that is, the higher the crystallinity index is the higher the activation energy. Actually, the activation energy for the pseudo-cellulose decomposition of the TORF sample is barely higher than that of the F sample, confirming that, in this case, essentially only the cellulose content is increased.
The increase in the cellulose content of the fibers and the crystallinity index, associated with higher activation energies, testifies an improved thermal resistance. Based on these considerations, it can be stated that the technical fibers are more flammable than the cleaned bast fibers whose behavior is, however, significantly affected by the decortication process. Mercerization is more effective than water washing for further improving the fiber thermal resistance, whereas mild torrefaction has a moderate effect essentially only on the cellulose content.
Conclusions
A thermogravimetric and kinetic analysis is made of the thermal degradation of hemp bast fibers obtained after water retting and mechanical decortication, hemp technical fibers, obtained after field retting, and hemp bast fibers subjected to hot water washing, very mild torrefaction and mercerization. The effects of manual versus mechanical decortication are also examined. The decomposition process is always well described by a four-step mechanism where the first two steps concern the more thermally labile non-cellulosic components, the third the pseudo-cellulose and the fourth the pseudo-lignin. The kinetic analysis shows that the activation energies of the non-cellulosic components (steps 1, 2, 4) are independent from the sample properties. Differences among samples can be well described by different activation energies for the pseudo-cellulose decomposition and the amounts of volatile released from the various reaction steps (small variations on the pre-exponential factors).
It has been observed that the volatile matter released from pseudo-cellulose decomposition increases from 54 mass% for the technical fibers up to a maximum of 80 mass% for the bast fibers subjected to the mercerization pretreatment with corresponding activation energies varying from about 211 to 249 kJ mol−1. The increase in the activation energy is generally associated with an increase in the crystallinity index. Hence, pretreatments modify both the content and properties of the fiber cellulose, indicating an improvement in the thermal resistance characteristics.
As expected, the less performing sample is the technical fiber, followed in the order by the fibers obtained by manual and mechanical decortication and finally by those subjected to the pretreatments of torrefaction, water washing and mercerization (the torrefaction pretreatment essentially causes only an increase in the cellulose content). The increase in the cellulose content is generally associated with a decrease in the more thermally labile non-cellulosic pseudo- components, especially that of the second step.
The in situ behavior of the hemp fiber cellulose is qualitatively and quantitatively close to that of cotton linter cellulose, provided the application of a four-step scheme also in this case. As already known, the decomposition of cotton linter is remarkably different from that of avicel cellulose (differences in purity level, molar mass distribution, average polymerization degree, amorphous portions of the structure, crystallite size). A different reaction scheme (a four-step for the CL vs. a single step for the avicel) results in approximately coincident activation energies (referred to the chief component of the CL releasing about 83 mass% volatile matter). Alternatively, the use of a single-step scheme results into different activation energies for the two samples (203 kJ mol−1 for the CL vs. 215 kJ mol−1 for the avicel).
References
Crini G, Lichtfouse E, Chanet G, Morin-Crini N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: a review. Environ Chem Lett. 2020;18:1451–76. https://doi.org/10.1007/s10311-020-01029-2.
Thygesen A, Madsen B, Bjerre AB, Lilholt H. Cellulosic fibers: effect of processing on fiber bundle strength. J Nat Fibers. 2011;8:161–75. https://doi.org/10.1080/15440478.2011.602236.
Cronier D, Monties B, Chabbert B. Structure and chemical composition of bast fibers isolated from developing hemp stem. J Agric Food Chem. 2005;53:8279–89. https://doi.org/10.1021/jf051253k.
Bousfield G, Morin S, Jacquet N, Riche A. Extraction and refinement of agricultural plant fibers for composites manufacturing. C R Chemie. 2018;21:897–906. https://doi.org/10.1016/j.crci.2018.07.001.
Placet V, Day A, Beaugrand J. The influence of unintended field retting on the physicochemical and mechanical properties of industrial hemp bast fibres. J Mater Sci. 2017;52:5759–77. https://doi.org/10.1007/s10853-017-0811-5.
Mazian B, Bergeret A, Bénézet JC, Malhautier L. Influence of field retting duration on the biochemical, microstructural, thermal and mechanical properties of hemp fibres harvested at the beginning of flowering. Ind Crop Prod. 2018;116:170–81. https://doi.org/10.1016/j.indcrop.2018.02.062.
Moriana R, Vilaplana F, Karlsson S, Ribes A. Correlation of chemical, structural and thermal properties of natural fibres for their sustainable exploitation. Carbohydr Polym. 2014;1121:422–31. https://doi.org/10.1016/j.carbpol.2014.06.009.
Zheng C, Li D, Ek M. Mechanism and kinetics of thermal degradation of insulating materials developed from cellulose fiber and fire retardants. J Therm Anal Calorim. 2019;135:3015–27. https://doi.org/10.1007/s10973-018-7564-5.
Wielage B, Lampke T, Marx G, Nestler K, Starke D. Thermogravimetric and differential scanning calorimetric analysis of natural fibres and polypropylene. Thermochim Acta. 1999;337:169–77. https://doi.org/10.1016/S0040-6031%2899%2900161-6.
Stevulova N, Estokova A, Cigasova J, Schwarzova I, Kacik F, Geffert A. Thermal degradation of natural and treated hemp hurds under air and nitrogen atmosphere. J Therm Anal Calorim. 2017;128:1649–60. https://doi.org/10.1007/s10973-016-6044-z.
Branca C, Di Blasi C, Galgano A. Experimental analysis about the exploitation of industrial hemp (Cannabis sativa) in pyrolysis. Fuel Process Technol. 2017;162:20–9. https://doi.org/10.1016/j.fuproc.2017.03.028.
Jakab E, Bora A, Sebestyen Z, Borsa J. Thermal decomposition of chemically treated cellulosic fibers. J Therm Anal Calorim. 2018;132:433–43. https://doi.org/10.1007/s10973-017-6935-7.
Rizhikovs J, Brazdausks P, Dobele G, Jurkjane V, Paze A, Meile K, Puke M. Pretreated hemp shives: possibilities of conversion into levoglucosan and levoglucosenone. Ind Crop Prod. 2019;139: 111520. https://doi.org/10.1016/j.indcrop.2019.111520.
Branca C, Di Blasi C. Thermal degradation behavior and kinetics of industrial hemp stalks and shives. Thermochim Acta. 2021;697: 178878. https://doi.org/10.1016/j.tca.2021.178878.
Freivalde L, Kukle S, Andzs M, Buksens E, Gravitis J. Flammability of raw insulation materials made of hemp. Compos B Eng. 2014;67:510–4. https://doi.org/10.1016/j.compositesb.2014.08.007.
Dorez G, Ferry L, Sonnier R, Taguet A, Lopez-Cuesta JM. Effect of cellulose, hemicellulose and lignin contents on pyrolysis and combustion of natural fibers. J Anal Appl Pyrolysis. 2014;107:323–31. https://doi.org/10.1016/j.jaap.2014.03.017.
Ouajai S, Shanks RA. Composition, structure and thermal degradation of hemp cellulose after chemical treatments. Polym Degrad Stab. 2005;89:327–35. https://doi.org/10.1016/j.polymdegradstab.2005.01.016.
Rachini A, Le Troedec M, Peyratout C, Smith A. Comparison of the thermal degradation of natural, alkali-treated and silane-treated hemp fibers under air and inert atmosphere. J Appl Polym Sci. 2009;112:226–34. https://doi.org/10.1002/app.29412.
Jeong H, Roh H, Lee J, Park J. Low-temperature pyrolysis on jute fibers as a thermochemical modification method. Fibers Polym. 2016;17:540–52. https://doi.org/10.1007/s12221-016-5879-z.
Czerniecka-Kubicka A, Janowski G, Pyda M, Frącz W. Biocomposites based on the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) matrix with the hemp fibers: thermal and mechanical properties. J Therm Anal Calorim. 2021. https://doi.org/10.1007/s10973-020-10492-6.
Sebestyen Z, May Z, Reczey K, Jakab E. The effect of alkaline pretreatment on the thermal decomposition of hemp. J Therm Anal Calorim. 2011;105:1061–9. https://doi.org/10.1007/s10973-010-1056-6.
Abdelouahed L, Leveneur S, Vernieres-Hassimi L, Balland L, Taouk B. Comparative investigation for the determination of kinetic parameters for biomass pyrolysis by thermogravimetric analysis. J Therm Anal Calorim. 2017;129:1201–13. https://doi.org/10.1007/s10973-017-6212-9.
Yeo JY, Chin BLF, Tan JK, Loh YS. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J Ener Inst. 2019;92:27–37. https://doi.org/10.1016/j.joei.2017.12.003.
Di Blasi C, Branca C, Galgano A, Gallo B. Role of pretreatments in the thermal runaway of hazelnut shell pyrolysis. Energy Fuels. 2015;29:2514–26. https://doi.org/10.1021/acs.energyfuels.5b00171.
Branca C, Di Blasi C, Galgano A. Pyrolytic conversion of wastes from cereal, protein and oil-protein crops. J Anal Appl Pyrolysis. 2017;127:426–35. https://doi.org/10.1016/j.jaap.2017.07.007.
Sfakiotakis S, Vamvuka D. Thermal decomposition behavior, characterization and evaluation of pyrolysis products of agricultural wastes. J Ener Inst. 2018;91:951–61. https://doi.org/10.1016/j.joei.2017.09.001.
Branca C, Di Blasi C, Elefante R. Devolatilization of conventional pyrolysis oils generated from biomass and cellulose. Energy Fuels. 2006;20:2253–61. https://doi.org/10.1021/ef0601059.
Branca C, Di Blasi C, Galgano A, Brostrom M. Effects of the torrefaction conditions on the fixed-bed pyrolysis of Norway spruce. Energy Fuels. 2014;28:5882–91. https://doi.org/10.1021/ef501395b.
Antal MJ, Várhegyi G, Jakab E. Cellulose pyrolysis kinetics: revisited. Ind Eng Chem Res. 1998;37:1267–75. https://doi.org/10.1021/ie970144v.
Martínez Gonzalez M, Marlin N, Da Silva Perez D, Dupont C, del Mar Saavedra Rios C, Meyer X-M, Gourdon C, Mortha G. Impact of cellulose properties on its behavior in torrefaction: commercial microcrystalline cellulose versus cotton linters and celluloses extracted from woody and agricultural biomass. Cellulose. 2021;28(8):4761–79. https://doi.org/10.1007/s10570-021-03812-y.
Kim UJ, Eom SH, Wada M. Thermal decomposition of native cellulose: Influence on crystallite size. Polym Degrad Stab. 2010;95:778–81. https://doi.org/10.1016/j.polymdegradstab.2010.02.009.
.JPS Morais M Freitas Rosa de M Moreira de Souza Filho de sá LD Nascimento DM Nascimento do A Ribeiro Cassales 2013 Extraction and characterization of nanocellulose structures from raw cotton linter Carbohydr Polym 91:229–235 https://doi.org/10.1016/j.carbpol.2012.08.010
Yu Y, Wu H. Significant differences in the hydrolysis behavior of amorphous and crystalline portions within microcrystalline cellulose in hot-compressed water. Ind Eng Chem Res. 2010;49:3902–9. https://doi.org/10.1021/ie901925g.
Branca C, Di Blasi C. A unified mechanism of the combustion reactions of lignocellulosic fuels. Thermochim Acta. 2013;565:58–64. https://doi.org/10.1016/j.tca.2013.04.014.
Branca C, Di Blasi C. Combustion kinetics of two core materials for sandwich structures. J Therm Anal Calorim. 2014;117:961–72. https://doi.org/10.1007/s10973-014-3845-9.
Branca C, Di Blasi C. Thermogravimetric analysis of the combustion of dry distiller’s grains with solubles (DDGS) and pyrolysis char under kinetic control. Fuel Proc Technol. 2015;129:67–74. https://doi.org/10.1016/j.fuproc.2014.08.019.
Moreno AI, Font R. Pyrolysis of furniture wood waste: decomposition and gases evolved. J Anal Appl Pyrolysis. 2015;113:464–73. https://doi.org/10.1016/j.jaap.2015.03.008.
Branca C, Di Blasi C. A summative model for the pyrolysis reaction heats of beech wood. Thermochim Acta. 2016;638:10–6. https://doi.org/10.1016/j.tca.2016.06.006.
Barta-Rajnai E, Várhegyi G, Wang L, Skreiberg Ø, Grønli M, Czégény Z. Thermal decomposition kinetics of wood and bark and their torrefied products. Energy Fuels. 2017;31:4024–34. https://doi.org/10.1021/acs.energyfuels.6b03419.
Font R. Potential kinetic model for thermal decomposition of complex organic compounds: significance of parameters and engineering application. Thermochim Acta. 2014;591:81–95. https://doi.org/10.1016/j.tca.2014.07.017.
Branca C, Di Blasi C, Horacek H. Analysis of the combustion kinetics and the thermal behavior of an intumescent system. Ind Eng Chem Res. 2002;41:2104–2014. https://doi.org/10.1021/ie010841u.
Varhegyi G, Wang L, Skreiberg Ø. Towards a meaningful non-isothermal kinetics for biomass materials and other complex organic samples. J Therm Anal Calorim. 2018;133:703–12. https://doi.org/10.1007/s10973-017-6893-0.
Branca C, Di Blasi C. Oxidation kinetics of chars generated from the acid-catalyzed pyrolysis of corncobs. Fuel Proc Technol. 2014;123:47–56. https://doi.org/10.1016/j.fuproc.2014.01.044.
Corradini E, Teixeira EM, Paladin PD, Agnelli JA, Silva ORRF, Mattoso LHC. Thermal stability and degradation kinetic study of white and colored cotton fibers by thermogravimetric analysis. J Therm Anal Calorim. 2009;97:415–9. https://doi.org/10.1007/s10973-008-9693-8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Branca, C., Di Blasi, C. Kinetic assessment of the thermal decomposition of hemp fiber and the impact of pretreatments. J Therm Anal Calorim 147, 14423–14435 (2022). https://doi.org/10.1007/s10973-022-11663-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11663-3