Abstract
This study aimed to use microcalorimetry to analyze the real-time effect that silver nitrate has on one of the most common bacteria involved in severe infections in current medical practice. Using a Calvet microcalorimeter, the heat output produced by Pseudomonas aeruginosa was determined when exposed to concentrations of silver nitrate from 0 to 3 mol⋅m−3, and a saturated solution. Under physiological human body temperature, for 46 h, thermographic curves were obtained. The maximum voltage peaks (Vmax) and the total amount of heat exchanged (Q) were determined. The results showed a bactericidal effect, with a minimum inhibitory concentration (MIC) of silver nitrate of 0.75 mol⋅m−3, showing a Vmax of 93 μV and Q of 193 kJ. With higher concentrations of this metallic solution, the results highlighted the proportional negative effect that silver nitrate has in Pseudomonas aeruginosa growth.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Biofilms of Pseudomonas aeruginosa are one of the most common and feared infections in current medical practice. This range of diseases includes chronic resistant periprosthetic infections, even in young or healthy patients after undergoing a joint replacement. This can happen despite all aseptic measures that current medicine offers. These infections often cannot be treated effectively with traditional antibiotic therapy and need further treatment. This also has an important impact to health care costs and therefore, there is a strong need for the development of more effective antibacterial agents and early diagnostic tools.
Infections by gram-negative non-fermenting rods, like Pseudomonas aeruginosa, that are frequently seen in orthopedic surgery and other fields [1], are expected to increase in the future. Especially if we consider the increasing number of elderly, diabetic [2], organ transplant and oncologic patients. According to the United States National Healthcare Safety Network, the infections caused by this type of bacteria rank fourth among the most important pathogens for all device-associated infections in the previous decade [3].
Silver has been known as an effective antimicrobial agent [4, 5]. Therefore, this and other metals have been used in various ways for their antimicrobial properties [6]. As a nanomaterial (Ag NPs) has been reported as agent with higher bactericide capacity than the chlorhexidine [7]. The latter is an antiseptic that is currently widely used in hospitals in the care of wounds and in the prophylactic skin preparation before surgeries. Besinis et al. [7] showed that Ag NPs had the strongest antibacterial activity of the NPs tested, with bacterial growth also being 25-fold lower than that in chlorhexidine.
It has been reported that silver nitrate prevents the separation into two daughter cells of sensitive dividing cells of Pseudomonas aeruginosa growing in nutrient broth [8]. In the pharmacopoeia of many countries, silver nitrate, together with silver itself, is used as an antiseptic and disinfectant applied topically [9]. It is also used as a cauterizer in superficial hemorrhages or to refresh sore ulcers, and it has been proposed as a coating material for endoprotheses and other implants in the surgical practice [10, 11]. This metal is an attractive material to be used as a component of fixation devices and joint prostheses in orthopedic surgery [12, 13], in order to decrease the postoperative infection rates.
Microcalorimetry is a technique that allows to determine, with high sensitivity, the energy exposed as consequence of any process or transformation [14, 15], which makes it a method of early detection to determine bacterial growth, from the energy exchanged in its metabolism [16]. It has been used in biology, pharmacology, biotechnology and ecology for its high sensitivity, precision and simplicity [17,18,19,20,21], however, clinical use has been very limited [22, 23].
The microcalorimetric analysis of bacterial species allows us to know in real time the growth curves of different microorganisms. Using this technique, the presence of bacteria in a culture medium can be confirmed in less than 10 h, even in samples with few colony-forming units (10 CFU⋅mL−1). These thermograms are characteristic of each bacterial species behaving like a “thermal fingerprint” and therefore, the bacterial species can be identified within a few hours of culture [24, 25]. Furthermore, from the behavioral patterns or “thermal fingerprint”, it is possible to extract the value of the specific parameters of each bacterium, such as growth constant, generation time and amount of heat exchanged [16, 26,27,28].
Microcalorimetric investigations of microbial processes are becoming increasingly popular. Some studies have focused on the study of bacterial growth as single microorganisms [19, 20, 24, 25, 29,30,31,32,33,34,35,36,37]. Moreover, it has been used to discriminate between strains of the same bacterial species; for example, to differentiate methicillin-sensitive strains of Staphylococcus aureus (MSSA) from methicillin-resistant strains of S. aureus (MRSA) [38].
Microcalorimetry has also been used as a tool to evaluate the growth and metabolism of bacterial cells in the face of changes in the composition of the culture medium [39,40,41,42,43] and to evaluate the effects of ultrasound waves on the viability of several bacteria that usually occur in mineral waters such as spa thermal waters [44].
In microbiology, the term “minimum inhibitory concentration” (MIC) is the lowest concentration of chemical, usually a drug, which inhibit visible growth of a bacteria. Currently, the MIC is used in antibiotics susceptibility testing. Meanwhile, the term “minimum bactericidal concentration” (MBC) is the minimum concentration of an antibacterial agent that results in bacterial death, therefore resulted in the inability to re-culture bacteria. The closer the MIC is to the MBC, the more bactericidal the compound.
The determination of antimicrobial activity of different drugs and toxic compounds has been carried out using calorimetry [19, 45,46,47,48,49], including de determination of the minimum inhibitory concentration (MIC) of antibiotics [49,50,51,52,53,54]. Esarte et al. [49] published the microcalorimetric behavior of Pseudomonas aeruginosa and its susceptibility against combination of antibiotics.
Regarding the medical field, microcalorimetry has been used for the detection of microbial contamination in platelet products, diagnosis of meningitis in cerebrospinal fluid of rats [22], detection of bacteria in artificial urine samples [50] or previously sterilized [51]. Furthermore, the microcalorimetry has shown to be a promising and efficient tool to investigate the formation of biofilms in dental implants [52] and bone grafts [53]. It has been used to study the antimicrobial efficacy of wound dressings containing antimicrobial agents [20] and of materials for implant coating [54].
Despite previous studies, there is lack of evidence of the effect of metallic solutions in bacteria growth. Aveledo et al. [55] reported the bacterial growth of Pseudomonas aeruginosa measured by microcalorimetry using different concentrations of zinc sulfate. The present paper is a continuation of that research whose objective is to analyze the bacterial growth of Pseudomonas aeruginosa under the exposure to silver nitrate.
Materials and methodology
The strains of bacteria that have been used were obtained from the American Type Culture Collection (ATCC) and the Spanish Type Culture Collection (CECT): Pseudomonas aeruginosa, ATCC 27,853. This bacterium was inoculated on blood agar plates and incubated at 309.65 K in an incubator for 24 h. The blood agar plates with multiple bacterial colonies were then used to prepare a bacterial suspension. Samples were prepared by adjusting turbidity concentration to 0.5 on the McFarland scale, using Colorimeter Vitek®, as an optical densimeter. This concentration was used to make further dilutions using 0.9% sterile saline solution (physiological), with national Code 641,860, to obtain final concentrations of 106 colony-forming units CFU⋅mL−1.
As culture medium we have used soy-casein broth digested with CO2, which is the most widely used culture medium in hospital clinical practice. The commercial presentation, BACTEC type PEDS PLUS/F, has the reference number 442194. US Pat. No. 4, 63,902; of National Committee for the Clinical Laboratory Standard (2001).
The metallic solutions used were provided by the Laboratory of Chemistry and Applied Physic of the University of Vigo. A saturated concentration of Silver nitrate was prepared, and from this solution, the different concentrations necessary for the experiments were prepared from 0 to 3 mol⋅m−3, and saturated solution (Fig. 1). Before each experience, these cells were washed with basic soap, passed through 96° alcohol and autoclaved at 121 °C for 30 min. In this order, sterile environment in the reference cell is guaranteed. Many autoclaves are used to sterilize equipment and supplies by subjecting them to pressurized saturated steam at 121 °C (249 °F) for around 15–20 min depending on the size of the load and the contents [56].
The measures were carried out in the Calvet microcalorimeter designed by Professor M. I. Paz Andrade [15]. It is equipped with a device allowing operation in the absence of vapor phase, and having two Teflon® screw capped stainless steel cells (experimental and reference) of approximately 10 cm3 [57]. In each experiment, both cells were introduced, from the upper part of the microcalorimeter into the internal thermopile chamber through two parallel aligned cylindrical holes (Fig. 2), which extended from the top of the microcalorimeter toward the internal thermopile chamber. The large distance that separates the cells from the inlet ensures the minimization of heat flow to the outside. As shown in Fig. 3, the reference cell was filled with 6 mL of culture medium, 1 mL of the metallic dissolution and 1 ml of mineral-medicinal water. In the experimental cell, the mineral-medicinal water was replaced by the bacterial suspension. At any time, a constant temperature of 309.65 K was maintained in the outer chamber of the calorimeter.
Structure of Calvet microcalorimeter [14]: (1) Cell access (2) Regulator system detector. (3) Heating resistance. (4) Aluminum tray. (5) Thermopiles. (6) Calorimetric block. (7) Equal heat distribution cone. (8) Multiple enclosure thermostat. (9) Direct heating
Experimental (left side) and reference cell (right side) placed inside the Calvet microcalorimeter internal block [14], adapted to our experience
The use of a Calvet microcalorimeter allows the monitoring of numerous physical, chemical and biological processes of long duration. The system used to collect data from the Calvet microcalorimeter is a Philips PM2535 multimeter, which registers the potential differences, connected through an RS232 interface to a data acquisition and control system consisting of a Raspberry PI minicomputer. Thus, using various modules, the design can be endowed with various functionalities. The operating system that runs on the minicomputer is LINUX, which provides the system with great robustness and reliability against failures. The precision in calorimetric signal achieved was ± 1 µV.
The heat voltage difference versus time was collected and recorded by the data acquisition and the processing system, at intervals of 22.2 s for approximately 48 h. The bacterial growth curves were obtained at different concentrations of the metallic dissolutions, represented as thermograms. The calorimetric curve is defined by the series of electromotive force points recorded by the data acquisition system, and corresponds to the energy exchange that occurs during the culture period. The differences in shape of curves allow us to visualize the trend of bacterial growth when increasing the concentration of the different metallic solutions. Therefore, a qualitative analysis can be made analyzing the aspect of the thermograms.
Samples were subjected to pH control, both before and after each experiment, using a basic 20 + pH meter, in order to measure the modification of the medium pH. In addition, this device shows the temperature of the sample that is being measured.
The maximum voltage peak (Vmax) was located in each thermograms, in order to carry out a quantitative analysis. Furthermore, the area under the curve (AUC) and the amount of heat exchanged (Q) during the time of the experiences of all the experiences were calculated. As the period of time of data collection varied between thermograms (46 to 52 h), the minimum time frame recorded of each group of metallic solution was used to calculate all the area under the curves (AUC), thus the total amount of heat exchanged (Q), for each group. In this way, we can make accurate comparisons by taking the data in a same period of time.
Using the data of the curves displayed by the microcalorimeter, the amount of heat released (Q) during the culture time can be determined using Eq. 1:
where A (μV⋅h) is the area under the curve (AUC), calculated by trapezoidal method, and K represents a constant, whose value of 23.8 μV−1⋅h−1 was calculated from the electric calibration performed by the Joule effect of the equipment [55]. A Setaram EJP30 stabilized current source was used to perform an electrical calibration.
Results and discussion
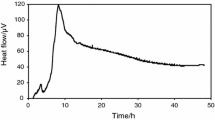
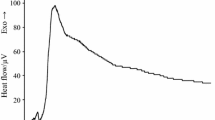
The results obtained are shown as heat flow versus time curves in Fig. 4. The bacterial growth of Pseudomonas aeruginosa follows a characteristic pattern when it is not exposed to any external agent. This growth pattern is represented in the thermogram of the concentration of 0 mol⋅m−3 (not added) of silver nitrate (Fig. 4a) [25]. The curve is characterized by the presence of one phase. It shows an initial ascending part with two main leaps. Then, a descending curve showing an exponential shape, which is prolonged over time. After 35 h, the changes of voltage signal were minimum, therefore the heat produced by bacteria metabolism remained almost without change on the last period of the experiment.
Calorimetric heat flow versus time for: a Pseudomonas aeruginosa [25], b P. aeruginosa + 0.75 mol⋅m−3 silver nitrate, c P. aeruginosa + 1 mol⋅m−3 silver nitrate, d P. aeruginosa + 1.5 mol⋅m−3 silver nitrate, e P. aeruginosa + 3 mol⋅m−3 silver nitrate, f P. aeruginosa + Saturated silver nitrate
The thermograms’ curves remained stable (no significant changes or effects were appreciated) when the bacteria were exposed to concentrations of silver nitrate lower than 0.75 mol⋅m−3. Therefore, this value was the minimum inhibitory concentration (MIC) of silver nitrate against this bacterial strain.
The maximum voltage peak (Vmax), the area under the curve (AUC46), and the amount of heat exchanged (Q46) during 46 h of the experiment of Pseudomonas aeruginosa with the different solutions of silver nitrate with the different solutions of silver nitrate that caused any effect on bacteria growth are shown in Table 1.
The voltage levels observed during these experiments were significantly low using higher concentrations of metallic dissolutions, with a strictly inverse proportionality with respect to the relation silver nitrate concentration/heat released. Among the thermograms obtained, the highest voltage peak (Vmax) was 119 μV using the bacterial suspension in the culture medium without the exposure of silver nitrate. Then, when increasing concentrations higher than 0.75 mol⋅m−3 were added, the voltage signal decreased gradually; showing a Vmax of 93 μV using a concentration of 0.75 mol⋅m−3, and 63 μV, 36 μV and 14 μV using concentrations of 1 mol⋅m−3, 1.5 mol⋅m−3 and 3 mol⋅m−3, respectively. No signal was detected when the saturated dissolution of silver nitrate was used. The total amount of heat exchanged showed the same pattern.
The total heat exchanged expressed as Q46, which was obtained using the area of the thermograms (AUC46) in Eq. 1, gradually decreased as the concentrations of silver nitrate increased. The sample with the bacteria alone produced 198 kJ, while the sample with 0.75 mol⋅m−3, 1 mol⋅m−3, 1.5 mol⋅m−3 and 3 mol⋅m−3 of silver nitrate, had a heat generation of 193 kJ, 112 kJ, 94 kJ and 11 kJ, respectively.
The shape of the thermograms during the experiments with silver nitrate (Fig. 4) showed similarity to the normal pattern of the growth of Pseudomonas aeruginosa only with the two lowest concentrations of this metallic dissolution; with the typical ascending phase with two main leaps, and the descending curve showing an exponential shape, which is prolonged over time. However, with higher concentrations of silver nitrate, where the bacteria growth was significantly inhibited, the bacteria growth curves did not follow the regular pattern. They did not show a sharp leap in the ascending curves. The concentration of 3 mol⋅m−3 of silver nitrate did not produce a prolonged exponential curve in the final phase, as no voltage signal was detected after 29 h of data collection. This was the minimum concentration of silver nitrate that caused total voltage suppression (0 μV) during the experiments, therefore, this concentration of silver nitrate corresponds to its minimum bactericidal concentration (MBC) against this bacterial strain.
The bactericidal effect of silver nitrate has already been reported, and our MIC and MBC values were similar to such published studies [58, 59]. However, according to the literature review performed, this is the first study that has assessed the bactericidal effect of silver nitrate using thermodynamic analysis.
Regarding the outcome of the temperature of the samples, measured before and at the end of the experiments, it increased in all experiences with different proportions (Table 2). The basal and final mean were 27.4 °C and 30.5 °C, respectively. This pattern was logically expected as, in order to recreate the physiological human body medium, a constant temperature of 309.65 K (36.5 °C) was maintained in the outer chamber of the calorimeter all the time. However, no significant differences or tendencies patterns were observed between the experiments using different concentrations of silver nitrate.
The outcome of the pH of the different samples after 46 h tended to increase or, in other words, they were more alkaline at the end of most experiences (Table 2). However, unlike the voltage signal/heat produced, the pH values were not proportional to the different concentrations of silver nitrate. Before starting this study, an overall acidifying effect was expected as the residues produced by the bacteria metabolism are usually acid, resulting in a decreased pH in culture medium. However, when reviewing the literature, studies have revealed the particular property of Pseudomonas aeruginosa to increase the pH when biofilm is produced [60].
Conclusions
Using microcalorimetry, this study highlighted the proportional negative effect that silver nitrate has in Pseudomonas aeruginosa growth. The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC), of this metallic solution against this bacterial strain, were defined. This thermodynamic technique supports the use of silver nitrate in the medical industry, among others, in order to take advantage of its bactericide property.
References
Jamei O, Gjoni S, Zenelaj B, Kressmann B, Belaieff W, Hannouche D, Uçkay I. Which Orthopaedic Patients Are Infected with Gram-negative Non-fermenting Rods? J Bone Joint Infect. 2017;2(2):73–6. https://doi.org/10.7150/jbji.17171.
Al-Mayahi M, Cian A, Kressmann B, de Kalbermatten B, Rohner P, Egloff M, Jafaar J, Malacarne S, Miozzari HH, Uçkay I. Associations of diabetes mellitus with orthopaedic infections. Infect Dis. 2016;48(1):70–3. https://doi.org/10.3109/23744235.2015.1082620.
Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. Antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. https://doi.org/10.1086/591861.
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52(4):662–8. https://doi.org/10.1002/1097-4636(20001215)52:4%3c662::aid-jbm10%3e3.0.co;2-3.
Lee D, Cohen RE, Rubner MF. Antibacterial properties of Ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir. 2005;21:9651–9. https://doi.org/10.1021/la0513306.
Hobman JL, Crossman LC. Bacterial antimicrobial metal ion resistance. J Med Microbiol. 2014;64:471–97. https://doi.org/10.1099/jmm.0.023036-0.
Besinis A, Peralta T, Handy RD. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology. 2014;8(1):1–16. https://doi.org/10.3109/17435390.2012.742935.
Richards RM. Antimicrobial action of silver nitrate. Microbios. 1981;31(124):83–91.
Ip M, Lui SL, Poon VKM, Lung I, Burd A. Antimicrobial activities of silver dressings: an in vitro comparison. J Med Microbiol. 2006;55:59–63. https://doi.org/10.1099/jmm.0.46124-0.
Gosheger G, Hardes J, Ahrens H, Streitburger A, Buerger H, Erren M, Gunsel A, Kemper FH, Winkelmann W, Von Eiff C. Silver-coated megaendoprostheses in a rabbit model–an analysis of the infection rate and toxicological side effects. Biomaterials. 2004;25(24):5547–56. https://doi.org/10.1016/j.biomaterials.2004.01.008.
Ewald A, Glückermann SK, Thull R, Gbureck U. Antimicrobial titanium/silver PVD coatings on titanium. BioMed Eng Online. 2006;5:22. https://doi.org/10.1186/1475-925X-5-22.
Song WH, Ryu HS, Hong SH. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation. J Biomed Mater Res A. 2009;88(1):246–54. https://doi.org/10.1002/jbm.a.31877.
Blaker JJ, Nazhat SN, Boccaccini AR. Development and characterisation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications. Biomaterials. 2004;25(7–8):1319–29. https://doi.org/10.1016/j.biomaterials.2003.08.007.
Calvet E, Prat H. Microcalorimétrie: Aplications physico-chimiques et biologiques. Paris: Masson el Cie Editeurs; 1956.
Paz Andrade MI. Les Dévelopements Récents de la Microcalorimétrie et de la Thermogenese. 1st ed. Paris: CRNS; 1967.
Boling EA, Blanchard GC, Russell WJ. Bacterial identification by microcalorimetry. Nature. 1973;241:472–3. https://doi.org/10.1038/241472a0.
O’Neill MAA, Vine GJ, Beezer AE, Bishop AH, Hadgraft J, Labetoulle C, Walter M, Bowler PG. Antimicrobial properties of silver-containing wound dressings: a microcalorimetric study. Int J Pharm. 2003;263(1–2):61–8. https://doi.org/10.1016/S0378-5173(03)00361-2.
Xu XJ, Xue Z, Xiao Q, Hou AX, Liu Y. Antibacterial Activities of Novel Diselenide-bridged bis(Porphyrin)s on Staphylococcus aureus Investigated by Microcalorimetry. Biol Trance Elem Res. 2008;125:185–92. https://doi.org/10.1007/s12011-008-8156-1.
Kong W, Wang J, Xing X, Jin C, Xiao X, Zhao Y, Zhang P, Zang P, Zang Q, Li Z. Screening for novel antibacterial agents based on the activities of compounds on metabolism of Escherichia coli: a microcalorimetric study. J Hazard Mater. 2011;185(1):346–52. https://doi.org/10.1016/j.jhazmat.2010.09.040.
Braissant O, Wirz D, Göpfert B, Daniela U. Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol Lett. 2010;303(1):1–8. https://doi.org/10.1111/j.1574-6968.2009.01819.x.
Maskow T, Kiesel B, Schubert T, Yong Z, Harms H, Yao J. Calorimetric real time monitoring of lambda prophage induction. J Virol Methods. 2010;168:126–32. https://doi.org/10.1016/j.jviromet.2010.05.002.
Trampuz A, Salzmann S, Antheaaume J, Daniela AU. Microcalorimetry: a novel method for detection of microbial contamination inplatelet products. Transfusión. 2007;47:1643–50. https://doi.org/10.1111/j.1537-2995.2007.01336.x.
Baldoni D, Hermann H, Frei R, Trampuz A, Steinhuber A. Performance of Microcalorimetry for Early Detection of Methicillin Resistance in Clinical Isolates of Staphylococcus aureus. J Clin Microbiol. 2009;47(3):774–6. https://doi.org/10.1128/JCM.02374-08.
Tan MR, Ren YS, Yan D, Meng XH, Cheng LH, Qiu L, Yan Y, Li J, Xiao X. Detection of microorganisms in different states based on microcalorimetry. J Therm Anal Calorim. 2012;109(2):1069–75. https://doi.org/10.1007/s10973-011-1804-2.
Lago N, Legido JL, Paz-Andrade MI, Arias I, Casas LM. Microcalorimetric study of the growth and metabolism of Pseudomonas aeruginosa. J Therm Anal Calorim. 2011;105:651–5. https://doi.org/10.1007/s10973-010-1175-0.
López D, Viñas M, Lorén JG, Bermúdez J. Analysis of microcalorimetric curves for bacterial identification. Can J Microbiol. 1987;33:6–11. https://doi.org/10.1139/m87-002.
Xiancheng Z, Zhaodong N, Yan X, Yuanqin Z, Honglin Z. Establishment of the power-time curve equation of bacterial growth in the log phase. J Therm Anal Calorim. 1999;58:279–85. https://doi.org/10.1023/a:1010194912678.
Vazquez C, Lago N, Mato MM, Legido JL, Esarte L. Microcalorimetric study of the growth of Enterococcus faecalis, Pseudomonas aeruginosa and their mixtures in an enriched culture medium. J Therm Anal Calorim. 2015;121(1):463–8. https://doi.org/10.1007/s10973-015-4606-0.
Belaich A, Belaich JP. Microcalorimetric study of the anaerobic growth of Escherichia coli: growth thermograms in a synthetic medium. J Bacteriol. 1976;125(1):14–8. https://doi.org/10.1128/jb.125.1.14-18.1976.
Xie CL, Tang HK, Song ZH, Qu SS, Liao YT, Liu HS. Microcalorimetric study of bacterial growth. Thermochim acta. 1988;123:33–41. https://doi.org/10.1016/0040-6031(88)80007-8.
Rodriguez BV, Contreras R, Almora E. Estudio microcalorimétrico del crecimiento de dos especies de micoplasmas. Interf Biotecnol. 1989;6(2):191–5.
Antoce AO, Dinu LD, Takahashi K. Microcalorimetry, a convenient technique for microbial growth monitoring. Roum Biotechnol Lett. 2001;6:243–51.
Maskow T, Babel W. Thermokinetic description of anaerobic growth of Halomonas halodenitrificans using a static microcalorimetric ampoule technique. J Biotechnol. 2003;101(3):267–74. https://doi.org/10.1016/S0168-1656(02)00341-3.
Ma J, Qi WT, Yang LN, Yu WT, Xie YB, Wang W, Ma XJ, Xu F, Sun LX. Microcalorimetric study on the growth and metabolism of microencapsulated microbial cell culture. J Microbiol Methods. 2007;68:172–7. https://doi.org/10.1016/j.mimet.2006.07.007.
Aerestrup FM, Hasman H. Susceptibility of different bacterial species isolated from food animals to copper sulphate, zinc chloride and antimicrobial substances used for disinfection. Vet Microbiol. 2004;100(1–2):83–9. https://doi.org/10.1016/j.vetmic.2004.01.013.
Zaharia DC, Iancu C, Steriade AT, Muntean AA, Balint O, Popa VT, Popa MI, Bogdan MA. MicroDSC study of Staphylococcus epidermidis growth. BMC Microbiol. 2010;10:322. https://doi.org/10.1186/1471-2180-10-322.
Bonkat G, Braissant O, Malte R, Solokhina A, Widmer AF, Frei R, Merwe A, Wyler S, Gasser TC. A Bachmann Standardization of isothermal microcalorimetry in urinary tract detection by using artificial urine. World J Urol. 2013;31(3):553–7. https://doi.org/10.1007/s00345-012-0913-2.
von Ah U, Wirz D, Daniels AU. Rapid differentiation of methicillin-susceptible Staphylococcus aureus from methicillin-resistant S. aureus and MIC determinations by isothermal microcalorimetry. J Clin Microbiol. 2008;46(6):2083–7. https://doi.org/10.1128/JCM.00611-08.
Ripa KT, Márdh PA, Hovelius B, Ljungholm K. Microcalorimetry as a tool for evaluation of blood culture media. J Clin Microbiol. 1977;5(4):393–6. https://doi.org/10.1128/jcm.5.4.393-396.1977.
Monk PR. Aerobic growth thermograms of Streptococcus lactis obtained with a complex medium containing glucose. J Bacteriol. 1978;135(2):373–8. https://doi.org/10.1128/jb.135.2.373-378.1978.
Dermoun Z, Belaich JP. Microcalorimetric study of Escherichia coli aerobic growth: kinetics and experimental enthalpy associated with growth on succinic acid. J Bacteriol. 1979;140(2):377–80. https://doi.org/10.1128/jb.140.2.377-380.1979.
Winkelmann M, Hütti R, Wolf G. Application of batch-calorimetry for the investigation of microbial activity. Thermochim Acta. 2004;415(1):75–82. https://doi.org/10.1016/j.tca.2003.08.028.
Kabanova N, Stulova I, Vilu R. Microcalorimetric study of the growth of bacterial colonies of Lactococcus lactis IL1403 in agar gels. Food Microbiol. 2012;29:67–79. https://doi.org/10.1016/j.fm.2011.08.018.
Liang H, Wu J, Liu Y, Yang L, Hu L, Qu S. Kinetics of the action of three selenides on Staphylococcus aureus growth as studied by microcalorimetry. Biol Trace Elem Res. 2003;92:181–7. https://doi.org/10.1385/BTER:92:2:181.
Wang X, Liu Y, Xie B, Shi X, Zhou J, Zhang H. Effect of nisin on the growth of Staphylococcus aureus determined by a microcalorimeric method. Mol Nutr Food Res. 2005;49(4):350–4. https://doi.org/10.1002/mnfr.200400079.
Hou AX, Xue Z, Liu Y, Qu SS, Wong WK. Antibacterial effects of a monoporphyrinato ytterbium (III) complex and its free components on Staphylococcus aureus as determined by stop-flow microcalorimetry. Chem Biodivers. 2007;4(7):1492–500. https://doi.org/10.1002/cbdv.200790128.
Xu XJ, Xue Z, Xiao Q, Hou AX, Liu Y. Antibacterial activities of novel diselenide-bridged bis(porphyrin)s on Staphylococcus aureus investigated by microcalorimetry. Biol Trace Elem Res. 2008;125:185–92. https://doi.org/10.1007/s12011-008-8156-1.
Dai C, Wang J, Kong W, Cheng P, Xiao X. Investigation of anti-microbial activity of catechin on Escherichia coli growth by microcalorimetry. Environ Toxicol Pharmacol. 2010;30(3):284–8. https://doi.org/10.1016/j.etap.2010.08.002.
Esarte L, Lago N, Vazquez C, Mato MM, Legido JL. Microcalorimetric method to determine ceftazidime and piperacillin–tazobactam susceptibility in Pseudomonas aeruginosa. J Therm Anal Calorim. 2015;121:353–60. https://doi.org/10.1007/s10973-015-4685-y.
Bonkat G, Braissant O, Rieken M, Solokhina A, Widmer AF, Frei R, Merwe A, Wyler S, Gasser TC, Bachmann A. Standardization of isothermal microcalorimetry in urinary tract infection detection by using artificial urine. World J Urol. 2013;31:553–7. https://doi.org/10.1007/s00345-012-0913-2.
Bonkat G, Braissant O, Widmer AF, Frei R, Rieken M, Wyler S, Gasser TC, Wirz D, Daniels AU, Bachmann A. Rapid detection of urinary tract pathogens using microcalorimetry: principle, technique and first results. BJU Int. 2012;110(6):892–7. https://doi.org/10.1111/j.1464-410X.2011.10902.x.
Astasov-Frauenhoffer M, Braissant O, Hauser-Gerspach I, Daniels AU, Weiger R, Waltimo T. Isothermal microcalorimetry provides new insights into biofilm variability and dynamics. FEMS Microbiol Lett. 2012;337(1):31–7. https://doi.org/10.1111/1574-6968.12007.
Clauss M, Trampuz A, Borens O, Bohner M, Ilchmann T. Biofilm formation on bone grafts and bone graft substitutes: comparison of different by a standard in vitro and microcalorimetry. Acta Biomater. 2010;6(9):3791–7. https://doi.org/10.1016/j.actbio.2010.03.011.
Baldoni D, Steinhuber A, Zimmerli W, Trampuz A. In vitro of Gallium maltolate against Staphylococci in logarithmic, stationary, and biofilm growth phases: comparison of conventional and calorimetric susceptibility testing methods. Antimicrob Agents Chemother. 2010;54(1):157–63. https://doi.org/10.1128/AAC.00700-09.
Aveledo R, Aveledo A, Vázquez C, Lago N, Mato MM, Legido JL. Study of bacterial sensitivity in zinc sulfate solutions by microcalorimetry. J Therm Anal Calorim. 2018;133:773–7. https://doi.org/10.1007/s10973-018-7000-x.
Black J. Microbiology. UK: Prentice Hall; 1993. p. 334.
Verdes PV, Mato MM, Paz Andrade MI, Legido JL. Contribution to study of the thermodynamics properties of mixtures containing 2-methoxy-2-methylpropane, alkanol, alkane. J Chem Thermodyn. 2014;73:224–31. https://doi.org/10.1016/j.jct.2014.01.002.
Pormohammad A, Greening D, Turner RJ. Synergism inhibition and eradication activity of silver nitrate/potassium tellurite combination against Pseudomonas aeruginosa biofilm. J Antimicrob Chemother. 2022;77(6):1635–44. https://doi.org/10.1093/jac/dkac094 (PMID: 35325152).
Pietsch F, Heidrich G, Nordholt N, Schreiber F. Prevalent Synergy and Antagonism Among Antibiotics and Biocides in Pseudomonas aeruginosa. Front Microbiol. 2021;11:615–8. https://doi.org/10.3389/fmicb.2020.615618.
Hunter R, Beveridge T. Application of a pH-Sensitive Fluoroprobe (C-SNARF-4) for pH Microenvironment Analysis in Pseudomonas aeruginosa. Biofilms Appl Environ Microbiol. 2005;71(5):2501–10. https://doi.org/10.1128/AEM.71.5.2501-2510.2005.
Acknowledgements
The authors are grateful to Miguel Ramos for the support in the preparations of the metallic dissolutions. Also, to Maria Perfecta Salgado Gonzalez for her collaboration with the technical measures. We thank for the financial support provided by the projects EM 2012/141, ED431C 2020/06 V055 and “Agrupación Estratégica de Biomedicina (INBIOMED)” by ‘‘Xunta de Galicia’’ of Spain.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
JLL conceived the original idea and supervised the project. NL, MMM helped supervise the project. JLL, NL, MMM planned the experiments. MMM wrote the manuscript with support from RA, JLL. RA prepared the samples, carried out the experiments, performed the calculations, designed the figures, analyzed the data and interpreted the results with support from MMM, NL, JLL. AA contributed to sample preparation. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aveledo, R., Aveledo, A., Lago, N. et al. Antibacterial activity of silver nitrate on Pseudomonas aeruginosa bacteria using Calvet calorimetry. J Therm Anal Calorim 148, 1639–1646 (2023). https://doi.org/10.1007/s10973-022-11603-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11603-1