Abstract
Pennes' bio-heat equation is the most widely used equation to analyze the heat transfer phenomenon associated with hyperthermia and cryoablation treatments of cancer. In this study, the semi-analytical and numerical solutions of Pennes' equation in a highly nonlinear form derived from renal cell carcinoma tissue's nonlinear specific heat capacity along with a freezing convection term were obtained and analyzed for the first time. Here, the governing equation was reduced to a lumped capacity form for simplification and exerted on a solid spherical renal tumor. In the following, two semi-analytical techniques, the adomian decomposition method (ADM) and the Akbari–Ganji's method (AGM) were evaluated in solving the governing ODE. The comparison revealed full conformity between ADM and AGM, in addition to an excellent agreement between the semi-analytical and the numerical results before the phase transition. The analysis highlighted a deviation between the semi-analytical and numerical results for the limited convergence of power-series-based semi-analytical methods throughout the phase change and beyond. For the investigated case with \(D_{t} = 0.025,\quad {\text{Biot}} = 0.075\), the convergence occurred while \(\tau \in [0,0.7]\) for both methods. Consequently, both semi-analytical techniques ADM and AGM, are applicable to find the solution of Pennes' bio-heat equation before the phase change, and there is no superiority in favor of one in accuracy. In contrast, numerical methods are reliable during the phase transition and after that. The analysis of the numerical solution showed that with the growth of the tumor, achieving the necrosis of the malignant tissue takes longer, and large tumors' temperature may not decrease to necrosis temperature. The interpretation of the numerical results indicated that cryoablation could be considered an effective thermal treatment for renal tumors with a diameter lower than 2.0 cm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pennes' bio-heat equation is the most common mathematical model to formulate biological systems with heat transfer [1]. Due to the extensive application of this equation in the mathematical modeling of the bio-heat transfer phenomenon, many attempts have been made to solve this well-known equation in different forms using analytical and numerical methods. The exact classical linear Pennes' equation solutions were obtained in one-dimensional multiregion Cartesian and spherical geometries with temperature-invariant physiological parameters by Durkee et al. In contrast, Liu and Tu investigated the bio-heat transfer phenomenon based on Pennes' equation considering transient blood temperature [2, 3]. In the following, Marin et al. did a finite element analysis (FEA) of the nonlinear Pennes' equation for skin tissue subjected to external thermal sources. Valente et al. implemented a computer simulation of hyperthermia with nanoparticles using the finite volume method (FVM). Ghazanfarian et al. discretized nonlinear Pennes' bio-heat equation, including the bio-heat transfer thermal wave model and the dual-phase-lag model using a mesh-free smoothed-particle hydrodynamics (SPH). Dehghani and Sabouri offered a spectral element method (SEM) for solving the Pennes' bio-heat equation through triangular and quadrilateral elements as a different numerical approach. Kalateh Bojdi and Askari Hemmat applied wavelet collection methods (WCMs) for solving Pennes' bio-heat equation [4]–[8]. Hatami et al. offered the heat transfer and fluid flow simulation of blood in the presence of a magnetic field analytically and numerically. Doosti et al. studied the uniform magnetic field effect on an electrically conduced material's melting rate and behavior. Ghalambaz et al. did a similar investigation for a nonuniform magnetic field [9]–[12]. Yue et al. derived a simplified one-dimensional bio-heat transfer model of cylindrical living tissues in a steady state. They offered the solution based on Bessel functions, whereas Al-Humedi and Al-Saadawi obtained a one-dimensional Pennes' equation solution numerically based on shifted Legendre polynomials. Lakhssassi et al. proposed a steady-state one-dimensional bio-heat equation based on the modified form of the Pennes' bio-heat equation, and Wang et al. obtained the analytical solution of one-dimensional Pennes' equation for the case of multiple electromagnetic heating pulses [13]–[16]. Giordano et al. achieved the fundamental solutions of the Pennes' bio-heat equation in rectangular, cylindrical and spherical coordinates. The solutions were used to solve a particular problem of magnetic fluid hyperthermia (MFH), and Zhang et al. similarly offered the method of fundamental solution (MFS) but coupled with the dual reciprocity method (DRM) to solve Pennes' equation in the steady form [17, 18]. Bedin and Bazán offered a series solution for a two-dimensional Pennes's equation with convective boundaries using the classical Fourier method [19]. Abdulhussein and Oda solved a time-fractional one-dimensional Pennes' equation, and Cui et al. gained the analytical solution of a fractional Pennes' bio-heat transfer equation on skin tissue applying the method of separation of variables, finite Fourier sine and the Laplace transform to solve the equation with the three common types of nonhomogeneous boundary conditions. Similarly, Qin and Wu presented a quadratic spline collocation method for the time-fractional Pennes' equation governing thermal therapy of tissues [20]–[22]. Zhang and Chauhan presented a cellular neural network (CNN) methodology as a real-time Pennes' equation to apply during the thermal ablation process [23]. A modified form of Pennes' equation to study the temperature distribution induced by any electrode array in an anisotropic tissue containing several nodules, including primary or metastatic with arbitrary shape, was proposed by Roca Oria et al. [24]. Zhao et al. created a two-level finite difference schema for one-dimensional Pennes's bio-heat equation. In the subsequent attempts, Luitel et al. extended the one-dimensional Pennes' equation by adding a protective clothing layer and derived the temperature profile at different time steps by employing the fully implicit finite difference method (FDM) [25, 26]. The solution of nonlinear bio-heat transfer equation in living tissues under periodic heat flux in tissue surface using the dual-phase lagging (DPL) non-Fourier heat conduction model and the adomian decomposition method (ADM) was obtained by Ghasemi et al. [27, 28]. Akbari et al. applied the Akbari–Ganji's method (AGM) to find the nonlinear governing equations solution of chemical reactors and also the arched beams with nonlinear vibration [29, 30]. Finding the semi-analytical solutions of Pennes' bio-heat equation and validation by comparing them with the numerical outputs will help adapt and improve these techniques to obtain more accurate closed-form answers according to the nonlinear characteristics of malignant tissues. On the other hand, analyzing the numerical solutions of Pennes' bio-heat equation gives an excellent perspective of tumors' behavior during thermal treatments. This study evaluates the capability of the two well-known semi-analytical techniques, the adomian decomposition method and the AGM, to obtain the solution of Pennes' bio-heat equation with a strong nonlinearity relevant to the nonlinear specific heat capacity of renal cell carcinoma tissue, in addition to finding the numerical solution and analyzing the results for the first time.
Material and methods
Hyperthermia treatment and cryoablation are two primary thermal methods to treat malignant tumors. Most developments of these two techniques are based on finding the solution of the Pennes' bio-heat transfer equation in different situations. The general form of Pennes' equation is [31]:
\(T,t,\rho_{\text t} ,c_{\text t} ,k,\rho_{\text b} ,c_{\text b} ,\omega_{\text p} ,T_{\text a} ,q_{\text m}\) are the tissue temperature, time, the density of the tissue, the specific heat capacity of the tissue, the thermal conductivity of the tissue, the density of blood, the specific heat capacity of blood, the blood perfusion rate, core body temperature and the volumetric metabolic heat generation rate, respectively.
Here the equation is reduced to a lumped capacity form for simplification purposes [32], and a cooling convection term is also added, as shown below:
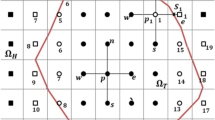
\(V_{\text t} ,h,A,T_{\infty }\) are the volume of tissue, convection coefficient, the area subjected to convection and the temperature of the convective medium. The term \(- \rho_{\text b} c_{\text b} \omega_{\text p} (T - T_{\text a} )\) shows the exit of energy via blood flow passing through the tumor. \(- hA(T(t) - T_{\infty } )\) presents the thermal energy exchange between the renal tumor and the surrounding environment with the temperature \(- 160{}^{^\circ }C\),which ablates the malignant cells. \(Q_{\text m}\) denotes the generated thermal energy by the metabolic reactions of a kidney. The purpose is to obtain \(T(t)\), which represents the temperature of the tumor subjected to the freezing environment over time. A schema of the problem is shown in Fig. 1.
By applying these new nondimensional parameters:
Equation (2) converts into the following nondimensional equation:
Considering core body temperature as the initial temperature (\(T_{0} = T_{\text a}\)(, Eq. (4) includes the following dimensionless initial condition:
The exact solution of Eq. (4) regarding the initial condition is:
Ponomarev and Pushkarev's experimental research demonstrated the temperature dependency of malignant renal tissue's specific heat capacity during cryoablation [33]. A two-term normal distribution with the following form was utilized to formulate this dependence and also cover the phase transition during this procedure:
which \(c_{\infty }\) indicates the specific heat capacity of the renal tumor at \(T_{\infty }\) and \(a_{1} ,a_{2} ,b_{1} ,b_{2} ,c_{1} ,c_{2}\) are constants. The numeric values of these constants are available in Table 1. The normal distribution associated with the specific capacity of renal cell carcinoma tumor is depicted in Fig. 2. It can be seen that near zero degrees Celsius, an intense increase in the value of specific heat capacity occurs, and it follows a nonlinear form. During a phase change, the temperature remains nearly constant at the freezing point, and all the exchanged thermal energy is consumed for the phase transition. After the phase transition termination, the specific heat capacity follows an almost linear function. By introducing the dimensionless groups below, the new form of (7) can be presented as (9).
Thus, the nondimensionalized form of the Pennes's bio-heat transfer equation with a convective term and nonlinear temperature-dependent specific heat capacity associated with malignant renal tissue will be in the form:
with the dimensionless initial condition.\(\theta (0) = \theta_{0} = 0\).
The numeric values of the physical parameters used to solve Pennes' equation exist in Table 2.
Here renal tumor is considered a solid sphere with the diameter \(D_{\text t}\) and the characteristic length \(L_{\text c}\) where:
Because:
and since the lumped analysis of a system is valid if only \(Biot < 0.1\)[38], then:
considering \(\theta (\tau ) = \frac{{T(t) - T_{a} }}{{T_{\infty } - T_{a} }}\) for the nondimensionalization of the governing Eq. (10), at:
Therefore, the values of \(\theta (\tau )\) vary from 0 to 1 throughout the freezing process.
Adomian decomposition method (ADM)
Adomian decomposition method (ADM) is a practical semi-analytical method based on infinite series to find the solution to nonlinear problems [39]. Exerting ADM on Eq. (10) as the governing equation of the problem leads [40]:
where \(L = \frac{d}{d\tau },N(\theta (\tau )) = \frac{{(\alpha_{1} + 1)\theta (\tau ) - (\alpha_{2} + 1)}}{{a_{1} e^{{ - (\beta_{1} \theta (\tau ) + \gamma_{1} )^{2} }} + a_{2} e^{{ - (\beta_{2} \theta (\tau ) + \gamma_{2} )^{2} }} }}.\) Applying \(L^{ - 1} = \int\limits_{0}^{\tau } {(.)d\tau }\) as the inverse.
operator on both sides of (10) and then applying the initial condition results:
Assuming the solution in the form of:
and expanding \(N(\theta (\tau ))\) as an infinite series in the form of adomian polynomials leads:
where:
Substituting (17), (18) into (16) ends in finding the approximate solution of (10).
Akbari–Ganji's method (AGM)
Akbari–Ganji's method (AGM) is a semi-analytical technique to find the solution of linear and nonlinear problems based on finite series. In this method, a finite series with unknown coefficients is considered to approximate the solution of the governing differential equation [41]. Applying the initial condition and perhaps the boundary conditions to the supposed solution, to the governing equation, and its derivatives after substituting the solution into the differential equation, an algebraic equation system appears which determines the unknown coefficients [42]-[43].
In AGM, the solution of (10) is assumed in the form:
Exerting the initial condition on (20) leads:
By inserting (20) into (10) and then applying the initial value to it, the following equation will emerge:
Substituting (21) into (22) will result \(\varphi_{1}\) as:
Through inserting (20) into (10) and deriving the outcome with respect to \(\tau\), and applying the initial condition and regarding the values of \(\varphi_{0}\) and \(\varphi_{1}\), \(\varphi_{2}\) will be obtained as:
Repeating this procedure \(n - 2\) times will compute the other unknown coefficients.
Numerical solution
The numerical methods that use the information at more than the last mesh point are referred to as multistep methods. The four-step Adams–Bashforth method and the modified Euler method as the starter technique were used to solve the governing Eq. (10) numerically. The solution was obtained based on the original variables \((t,T(t))\) for the various values of \(h\) and Biot number.
Results and discussion
Adomian decomposition method
The first sixteen terms of the solution for \(D_{t} = 0.025(m)\) and \(Biot = 0.075\) using ADM are expressed as follows:
Figure 3 displays the semi-analytical solution of Pennes' equation obtained by ADM (25) in comparison to the numerical solution. As it can be seen, there is a perfect agreement between ADM and the numerical solution before the phase transition. The reason for the deviation after the phase change is the limited convergence domain of power-series-based methods, which is [0,0.7] for the investigated case here.
Solution of Eq. (10) with \(D_{\text t} = 0.025(m)\) and \(Biot = 0.075\),\(n = 16\)
Numerical method
The first sixteen terms of the solution for \(D_{t} = 0.025(m)\) and \(Biot = 0.075\) applying AGM are denoted as below:
Figure 4 displays the semi-analytical solution of Pennes' equation obtained by AGM (26) in comparison to the numerical solution. As it can be seen, there is a perfect agreement between AGM and the numerical solution before the phase transition. AGM also includes a limited convergence domain similar to other power-series-based semi-analytical methods such as ADM, and the convergence domain is [0,0.7] for the studied case.
Solution of Eq. (10) with \(D_{t} = 0.025(m)\) and \(Biot = 0.075\),\(n = 16\)
As observed, in all the investigated cases, the temperature of the tumor falls from \(37\;^\circ {\text{C}}\)(core body temperature) and approaches the convection medium's temperature \(- 160\;^\circ {\text{C}}\) during cryoablation. However, the duration of this process increases with the growth of tumors' diameter. Consequently, larger tumors need more time to ensure their complete necrosis. Moreover, with the growth of the tumor’s diameter, its temperature becomes stable at a higher value, and it does not reach the essential magnitude to guarantee necrosis. Therefore, it appears that the acceptable diameter to treat a renal cell carcinoma tumor through cryoablation is \(D_{\text t} \le 0.02(m)\). The numerical method was implemented for the tumor’s diameters = 0.01, 0.015, 0.02, 0.025, 0.03 (m) and the Biot numbers = 0.025, 0.05, 0.075, 0.1. These numerical solutions are illustrated in Fig. 5.
Conclusions
Considering the solutions of the two semi-analytical methods, ADM and AGM, it is evident that the outputs of these two methods are entirely coincident. As observed in Figs. 3 and 4, the convergence domain of both methods is from the starting time to the phase transition. For \(D_{\text t} = 0.025,\quad {\text{Biot}} = 0.075\) this interval is nearly \(\tau \in [0,0.7]\). The semi-analytical methods and the numerical technique show excellent agreement in this domain. It approves that ADM and AGM are both suitable methods to solve the nonlinear form of the Pennes' bio-heat transfer equation before phase transition, while numerical techniques are still the appropriate procedures to find the solution during phase change and beyond. This is because of the limited convergence domain of power-series-based semi-analytical methods, which is a common characteristic. The convergence domain is limited to the phase transition for these two techniques. Comparing the numerical solutions determines that with the increase in the diameter of renal carcinoma tumors, approaching the suitable temperature for cryoablation takes longer. The results also confirm that cryoablation can effectively treat tumors with a diameter lower than 0.02(m).
References
Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1(2):93–122. https://doi.org/10.1152/jappl.1948.1.2.93.
Durkee JW, Antich PP, Lee CE. Exact solutions to the multiregion time-dependent bioheat equation. II: numerical evaluation of the solutions. Phys Med Biol. 1990;35(7):869–89. https://doi.org/10.1088/0031-9155/35/7/005.
Liu KC, Tu FJ. Numerical solution of bioheat transfer problems with transient blood temperature. Int J Comput Methods. 2019;16(4):1–12. https://doi.org/10.1142/S0219876218430016.
Marin M, Hobiny A, Abbas I. Finite element analysis of nonlinear bioheat model in skin tissue due to external thermal sources. Mathematics. 2021;9(13):1–9. https://doi.org/10.3390/math9131459.
Valente A, Loureiro F, Di Bartolo L, Mansur WJ. Computer simulation of hyperthermia with nanoparticles using an OcTree finite volume technique. Int Commun Heat Mass Transf. 2018;91:248–55. https://doi.org/10.1016/j.icheatmasstransfer.2017.12.021.
Ghazanfarian J, Saghatchi R, Patil DV. Implementation of Smoothed-Particle Hydrodynamics for non-linear Pennes’ bioheat transfer equation. Appl Math Comput. 2015;259:21–31. https://doi.org/10.1016/j.amc.2015.02.036.
Dehghan M, Sabouri M. A spectral element method for solving the Pennes bioheat transfer equation by using triangular and quadrilateral elements. Appl Math Model. 2012;36(12):6031–49. https://doi.org/10.1016/j.apm.2012.01.018.
Bojdi ZK, Hemmat AA. Wavelet collocation methods for solving the Pennes bioheat transfer equation. Optik (Stuttg). 2017;130:345–55. https://doi.org/10.1016/j.ijleo.2016.10.102.
Hatami M, Hatami J, Ganji DD. Computer simulation of MHD blood conveying gold nanoparticles as a third grade non-Newtonian nanofluid in a hollow porous vessel. Comput Methods Programs Biomed. 2014;113(2):632–41. https://doi.org/10.1016/j.cmpb.2013.11.001.
Doostani A, Ghalambaz M, Chamkha AJ. MHD natural convection phase-change heat transfer in a cavity: analysis of the magnetic field effect. J Brazilian Soc Mech Sci Eng. 2017;39(7):2831–46. https://doi.org/10.1007/s40430-017-0722-z.
M. Ghalambaz, S. M. Hashem Zadeh, S. A. M. Mehryan, K. Ayoubi Ayoubloo, and N. Sedaghatizadeh, “Non-Newtonian behavior of an electrical and magnetizable phase change material in a filled enclosure in the presence of a non-uniform magnetic field,” Int. Commun. Heat Mass Transf., 2020; 110:104437. Doi: https://doi.org/10.1016/j.icheatmasstransfer.2019.104437.
Ghalambaz M, Sabour M, Sazgara S, Pop I, Trâmbiţaş R, Insight into the dynamics of ferrohydrodynamic (FHD) and magnetohydrodynamic (MHD) nanofluids inside a hexagonal cavity in the presence of a non-uniform magnetic field. J Magn Magn Mater., 2019; p. 166024. Doi: https://doi.org/10.1016/j.jmmm.2019.166024.
Yue K, Zhang X, Yu F. Analytic solution of one-dimensional steady-state Pennes’ bioheat transfer equation in cylindrical coordinates. J Therm Sci. 2004;13(3):255–8. https://doi.org/10.1007/s11630-004-0039-y.
Al-Humedi HO, Al-Saadawi FA. The numerical solution of bioheat equation based on shifted legendre polynomial. Int J Nonlinear Anal Appl. 2021;12(2):1061–70. https://doi.org/10.22075/ijnaa.2021.5175.
Lakhssassi A, Kengne E, Semmaoui H. Modifed pennes’ equation modelling bio-heat transfer in living tissues: analytical and numerical analysis. Nat Sci. 2010;02(12):1375–85. https://doi.org/10.4236/ns.2010.212168.
Wang H, Burgei WA, Zhou H. Analytical solution of one-dimensional Pennes’ bioheat equation. Open Phys. 2020;18(1):1084–92. https://doi.org/10.1515/phys-2020-0197.
Giordano MA, Gutierrez G, Rinaldi C. Fundamental solutions to the bioheat equation and their application to magnetic fluid hyperthermia. Int J Hyperth. 2010;26(5):475–84. https://doi.org/10.3109/02656731003749643.
Zhang ZW, Wang ZW, Qin QH, Method of fundamental solutions for nonlinear skin bioheat model. J Mech Med Biol. 2014; 14(4). Doi: https://doi.org/10.1142/S0219519414500602.
Bedin L, Bazán FSV. On the 2D bioheat equation with convective boundary conditions and its numerical realization via a highly accurate approach. Appl Math Comput. 2014;236:422–36. https://doi.org/10.1016/j.amc.2014.03.071.
Abdulhussein AM, Oda H, The numerical solution of time-space fractional bioheat equation by using fractional quadratic spline methods. AIP Conf Proc, 2020; 2235. May, 2020. Doi: https://doi.org/10.1063/5.0007692.
Cui ZJ, Chen GD, Zhang R. Analytical solution for the time-fractional pennes bioheat transfer equation on skin tissue. Adv Mater Res. 2014;1049–1050:1471–4. https://doi.org/10.4028/www.scientific.net/AMR.1049-1050.1471.
Qin Y, Wu K. Numerical solution of fractional bioheat equation by quadratic spline collocation method. J Nonlinear Sci Appl. 2016;09(07):5061–72. https://doi.org/10.22436/jnsa.009.07.09.
Zhang J , Chauhan S, Neural network methodology for real-time modelling of bio-heat transfer during thermo-therapeutic applications. Artif. Intell. Med. 2019;101: 101728. July 2019. Doi: https://doi.org/10.1016/j.artmed.2019.101728.
Roca Oria EJ, Cabrales EB, Bory Reyesc J, Analytical solution of the bioheat equation for thermal response induced by any electrode array in anisotropic tissues with arbitrary shapes containing multiple-tumor nodules. Rev Mex Fis. 2019; 65(3), 284–290. Doi: https://doi.org/10.31349/RevMexFis.65.284.
Zhao JJ, Zhang J, Kang N, Yang F. A two level finite difference scheme for one dimensional Pennes’ bioheat equation. Appl Math Comput. 2005;171(1):320–31. https://doi.org/10.1016/j.amc.2005.01.052.
Luitel K, Gurung DB, Khanal H, Uprety KN, Bioheat Transfer Equation with Protective Layer. Math Probl Eng. 2021. Doi: https://doi.org/10.1155/2021/6639550.
Hadi Ghasemi M, Hoseinzadeh S, Memon S, A dual-phase-lag (DPL) transient non-Fourier heat transfer analysis of functional graded cylindrical material under axial heat flux. Int Commun Heat Mass Transf. 2022;131. Doi: https://doi.org/10.1016/j.icheatmasstransfer.2021.105858
Ghasemi A, Dardel M. Mohammad Hassan Ghasemi, “collective effect of fluid’s colioris force and nano-scale’s parameter on instability pattern and vibration characteristic of fluid-conveying carbon nanotubes.” J Pressure Vessel Technol. 2015;137(3): 031301. https://doi.org/10.1115/1.4029522.
Sara A, Esmaeil K, Akbari-Ganjis method AGM to chemical reactor design for non-isothermal and non-adiabatic of mixed flow reactors. J Chem Eng Mater Sci. 2020; 11(1), 1–9. Doi: https://doi.org/10.5897/jcems2018.0320.
Akbari MR, Ganji DD, Ahmadi AR, et al. Analyzing the nonlinear vibrational wave differential equation for the simplified model of Tower Cranes by Algebraic Method. Front Mech Eng. 2014;9:58–70. https://doi.org/10.1007/s11465-014-0289-7.
Becker SM, Chapter 4 - Analytical Bioheat Transfer: Solution Development of the Pennes’ Mode. ” In, Becker SM, Kuznetsov AV, (eds.) Heat transfer and fluid flow in biological processes Academic Press, Boston 2015; 77–124. Doi: https://doi.org/10.1016/B978-0-12-408077-5.00004-3.
Hristov J, Bio-heat models revisited: concepts, derivations, nondimensalization and fractionalization approaches. Front. Phys. 2019; 7. November, 2019. Doi: https://doi.org/10.3389/fphy.2019.00189.
Ponomarev DE, Pushkarev AV, Research of human kidney thermal properties for the purpose of cryosurgery. J Phys Conf Ser., 2017;891(1). Doi: https://doi.org/10.1088/1742-6596/891/1/012336.
He X, Bischof JC. Analysis of thermal stress in cryosurgery of kidneys. J Biomech Eng. 2005;127(4):656–61. https://doi.org/10.1115/1.1934021.
Johar RS, Smith RP. Assessing gravimetric estimation of intraoperative blood loss. J Gynecol Surg. 1993;9(3):151–4. https://doi.org/10.1089/gyn.1993.9.151.
Das CJ. Perfusion computed tomography in renal cell carcinoma. World J Radiol. 2015;7(7):170. https://doi.org/10.4329/wjr.v7.i7.170.
Orlande HRB, Lutaif NA, Gontijo JAR. Estimation of the kidney metabolic heat generation rate. Int j numer method biomed eng. 2019;35(9):1–19. https://doi.org/10.1002/cnm.3224.
Hahn MN, Özişik DW, Heat Conduction Fundamentals. In Heat Conduction, 3rd ed., John Wiley & Sons, Ltd, 2012; pp. 1–39. Doi: https://doi.org/10.1002/9781118411285.ch1.
Adomian G. A review of the decomposition method and some recent results for nonlinear equations. Math Comput Model. 1990;13(7):17–43. https://doi.org/10.1016/0895-7177(90)90125-7.
Adomian Decomposition Method. In Advanced numerical and semi‐analytical methods for differential equations. John Wiley & Sons, Ltd., 2019, pp. 119–130. Doi: https://doi.org/10.1002/9781119423461.ch11.
Tahernejad Ledari S, Domiri Ganji D, Mirgolbabaee H, An assessment of a semi analytical AG method for solving nonlinear oscillators. New Trends Math. Sci., 2016; 4(1):283–283. Doi: https://doi.org/10.20852/ntmsci.2016116028.
Hoseinzadeh S, Sohani A, Ashrafi TG. An artificial intelligence-based prediction way to describe flowing a Newtonian liquid/gas on a permeable flat surface. J Therm Anal Calorim. 2022;147:4403–9. https://doi.org/10.1007/s10973-021-10811-5.
Hoseinzadeh S, Heyns PS, Chamkha AJ, et al. Thermal analysis of porous fins enclosure with the comparison of analytical and numerical methods. J Therm Anal Calorim. 2019;138:727–35. https://doi.org/10.1007/s10973-019-08203-x.
Acknowledgements
The professional graphic design assistance provided by Ms. Najmeh Kamali Dehghan is greatly appreciated.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ostadhossein, R., Hoseinzadeh, S. The solution of Pennes' bio-heat equation with a convection term and nonlinear specific heat capacity using Adomian decomposition. J Therm Anal Calorim 147, 12739–12747 (2022). https://doi.org/10.1007/s10973-022-11445-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11445-x