Abstract
The effects of covalently bound phosphorus (P-) and nitrogen (N-) bearing groups on the thermal and combustion attributes of polystyrene have been investigated. The necessary chemical modifications were achieved through co- and ter-polymerisation reactions, in a suitable solvent, under radical initiation conditions. The influence of P–N cooperative interactions on the combustion properties of styrenic polymers was studied. The co-monomers of interest included: diethyl(acryloyloxymethyl)phosphonate (DEAMP), diethyl-p-vinylbenzylphosphonate (DEpVBP), acrylic acid-2-[(diethoxyphosphoryl)methyl amino]ethyl ester (ADEPMAE) and maleimide (MI). For the first time, the ter-polymers of styrene containing both P- groups, DEAMP or DEpVBP, and N- groups, MI, were prepared via solution polymerisation. It was found that the thermal stability and combustion characteristics of polystyrene were significantly altered by the presence of nominal amounts of P- and N- containing groups, and, in certain cases, cooperative interactions of these groups were also evident. For instance, the extents of char formation post-degradation of the prepared ter-polymers, as revealed by thermogravimetric investigations in an inert atmosphere (nitrogen), were found to be enhanced by more than 20%, as compared to the unmodified polystyrene. The heat release rates and heat release capacities of the ter-polymers, as measured using the pyrolysis combustion flow calorimetric (PCFC) technique, were reduced by almost 50% in comparison to the same parameters obtained for the unmodified counterpart.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polystyrene (PS) is a well-known thermoplastic polymer, which is extensively used for various applications, especially as an insulation material in the building sector [1]. However, PS owing to its relatively high flammability, has an increased propensity for ignition that often leads to a rapid and uncontrolled fire spread. Furthermore, styrenic polymers generally undergo combustion processes that are associated with the generation of significant amounts of smoke and nearly zero amounts of char [2]. Therefore, a wide range of fire retardants (FRs) have been developed to improve the fire resistance of PS-based products. Among them, the halogenated FRs dominated the polymer industry in the past due to their high effectiveness and low production costs. However, recently, the application of this class of FRs has been restricted, or partially phased out, in many countries due to their toxicity and bioaccumulation issues, which, in turn, has led to the rapid development of non-halogenated FR formulations [3].
Among the halogen-free FR options, phosphorus (P-)-based compounds are found to be relatively less toxic yet effective [4]. Several studies carried out in recent years have been focused on P- containing compounds acting as FRs for styrenic polymers, including red phosphorus, phosphine oxides, inorganic and organic phosphates, or blends of P- containing compounds with other FRs [5]. However, relatively high loadings (20–40 mass%) of additive FRs are normally required to achieve acceptable levels of fire retardance. In most instances, this could result in detrimental changes of physical and mechanical properties of the parent polymeric matrix. Thus, as an alternative (i.e. reactive FRs), certain P- containing compounds can be used for the chemical modification of PS via a radical polymerisation technique [5]. Among the various P- containing reactive FRs for PS, unsaturated organophosphorus compounds are considered to be the preferred options, as they can be chemically bonded to the polymeric chains through the chain-growth processes [6]. In recent years, different P- containing FRs have been incorporated into the main chain of PS. These materials displayed a significant increase of the limiting oxygen index (LOI) along with the increase of residual char yields, in comparison to the same parameters for the homo-polymer, PS [6,7,8,9,10,11,12]. With a view to obtaining better levels of fire retardance, the feasibility of using formulations, which combine P- containing FRs with other heteroatom-bearing compounds has been explored. In the case of PS, among the various systems explored so far, the reactive FRs with P- and N- containing moieties were found to be effective [9, 13,14,15,16]. However, there has been no reports that systematically studied the effects of different chemical environments within P- and N- groups on the thermal stability and combustion characteristics of styrenic polymers. Moreover, ter-polymerisation reactions of styrene (S) with P- and N- bearing monomers have not been attempted at all in the past, and hence warrant further useful investigations.
In the present study, three unsaturated compounds containing P atom in different chemical environments have been synthesised. These include diethyl(acryloyloxymethyl)phosphonate (DEAMP), diethyl-p-vinylbenzylphosphonate (DEpVBP) and acrylic acid-2-[(diethoxyphosphoryl)methyl amino]ethyl ester (ADEPMAE). The impacts of these monomeric units on the thermal/combustion behaviours of polyacrylonitrile (PAN) and polymethyl methacrylate (PMMA) have been previously reported [17]. In this work, the base polymer, PS, was chemically modified through a solution polymerisation route. For comparing the effects of N-containing groups on the combustion behaviour of PS, different N-containing compounds such as maleimide (MI), acrylamide (AM) and dimethyl acrylamide (DMA) were used as the monomers for the preparation of styrene-based co-polymers (S). The above-mentioned P- monomers and the N-containing unsaturated compound, MI, were selected from the initial screening tests for combustion characteristics of the corresponding co-polymers. Furthermore, for the first time, DEAMP, DEpVBP and MI were used in the preparation of styrene-based ter-polymers such as poly(S-ter-DEAMP-ter-MI) and poly(S-ter-DEpVBP-ter-MI). A comparison of cooperative effects has been attempted in the case when P- and N- atoms are present within the same pendent group (as in the co-polymer, poly(S-co-ADEPMAE), or when these atoms are in different modifying groups (as in the ter-polymers, poly(S-ter-DEAMP-ter-MI) and poly(S-ter-DEpVBP-ter-MI). The thermal stabilities and combustion characteristics of homo-, co- and ter- polymers were determined using different techniques, such as: Thermogravimetric Analysis (TGA), Differential Scanning Calorimetry (DSC), Pyrolysis Combustion Flow Calorimetry (PCFC) and ‘Bomb’ Calorimetry.
Experimental
Materials
The used P- and N- containing monomers include: diethyl(acryloyloxymethyl)phosphonate (DEAMP) (I), diethyl-p-vinylbenzylphosphonate (DEpVBP) (II), acrylic acid-2-[(diethoxyphosphoryl)methyl amino]ethyl ester (ADEPMAE) (III) and maleimide (MI) (IV). The structures of the monomers are shown in Fig. 1. The synthetic procedures for the P- containing monomers (I-III) were reported elsewhere [12, 18, 19]. All chemicals and reagents were obtained from Merck Company (UK). The solid compounds were used as received, whereas liquid reagents and organic solvents were dried over molecular sieves (4 Å). Styrene containing 10–15 ppm of 4-ter-butylcatechol (inhibitor) was purified by passing it through a proprietary inhibitor removal column.
Preparation of styrene-based polymers
Homo-, co-, and ter- polymers of styrene were prepared by radical solution polymerisation using toluene or N, N-dimethylformamide (DMF) as solvents, depending on the solubilities of the monomers. In all the cases, azobisisobutyronitrile (AIBN) was used as an initiator, with a concentration of ca. 2 g L−1. The synthetic procedure for the preparation of PS, co- and ter- polymers was as follows:
An accurately measured mass of styrene (S) (or a mixture of styrene with the monomers (I-IV) as shown in Table 1) was placed in a three-necked round-bottomed flask, fitted with a magnetic stirrer, a water condenser, and a bubbler. The monomers were added dropwise to the solvent, which has been previously flushed with argon at room temperature for at least 30 min. The reaction mixture was stirred for ca. 30 min with argon bubbling through it at room temperature, and then slowly heated to 60 ± 0.2 °C. Once this temperature was reached, AIBN dissolved in the solvent was added dropwise to the reaction mixture. The polymerisation was allowed to proceed for 16 h under a blanket of argon. After the required reaction time, the resulting polymers were recovered by precipitation in a five-fold excess of a non-solvent (methanol). Subsequently, the precipitated polymeric materials (white powders) were collected by filtration at reduced pressure and washed with methanol several times to remove any unreacted monomers. The polymers, after the initial drying in a vacuum oven, were purified by precipitation from their solutions (in dichloromethane (DCM), or DMF) into the non-solvent. After filtration, the obtained products were dried in a vacuum oven at 50 ± 1 °C for 16 h before further examinations.
Characterisation techniques
Fourier Transform Infrared (FT-IR) spectroscopy of the polymers in the finely powdered forms was carried out in the Attenuated Total Reflectance (ATR) mode using a Thermo Nicolet, Nexus spectrometer (Nicolet, USA). The spectra were run (64 scans) over a wavenumber range of 4000–400 cm−1 and with a resolution of 4 cm−1.
1H NMR of polymers were recorded in deuterated solvents (chloroform (CDCl3), or DMF) using a Bruker spectrometer (Bruker, Coventry, UK), operating at 600 MHz for protons. The 1H NMR spectra of each polymer were used to calculate the degree of incorporation of P- and N- containing monomeric units, and subsequently the P and N loadings (mass %) [17].
Thermogravimetric analysis (TGA) was performed using a PerkinElmer, Pyris 1 TGA (Beaconsfield, UK) instrument according to BS EN ISO 11358-1: 2014. The TGA runs were carried out on ca. 8 mg sample of a monomer or a polymer, at a heating rate of 10 °C min−1 under both nitrogen and air atmospheres, and in the temperature interval between 30 and 800 °C. The TGA tests of polymer samples were also carried out at 60 °C min−1 under nitrogen between 30 and 800 °C for comparing the results with those obtained from PCFC (heating rate of 60 °C min−1). All samples were tested in duplicates to ensure the repeatability.
Differential Scanning Calorimetry (DSC) was carried out using a Mettler Toledo DSC1/700 instrument (Leicester, UK). Each sample in a powdered form (ca. 8 mg) was placed in a standard aluminium DSC crucible with a hole in the lid, and heated from 30 to 500 °C, under nitrogen atmosphere, at a heating rate of 10 °C min−1 and at a flow rate of 50 mL min−1.
Bomb calorimetric runs were performed using a Parr 6200 calorimeter to determine the heat of combustion in accordance with BS EN ISO 18125:2017. The measurements were conducted on samples, in the form of a pellet, weighing ca. 0.5 g. The ‘bomb’ was filled with oxygen up to a pressure of 31 bars and ignited. For each sample, triplicate runs were done for better accuracy and the average values were presented.
Pyrolysis Combustion Flow Calorimetric (PCFC) measurements were taken using a Fire Testing Technology Ltd. (Gosport, UK) micro-scale combustion calorimeter according to ASTM D7309. For each run, an accurately weighed sample was firstly heated to about 900 °C at a heating rate of ca. 60 °C min−1, in a stream of nitrogen. The thermal degradation products were collected and then mixed with a stream of air prior to entering a combustion chamber maintained at 900 °C. All the tests were run in triplicates, and the average values were calculated.
Results and discussion
The homo-polymer and corresponding chemically modified polymers of styrene were prepared through solution polymerisation technique under radical initiation. The synthetic scheme pertaining to the chemical routes to the modification of PS is given in Fig. 2. The styrene-based polymers (i.e., homo-, co- and ter- polymers) were obtained in the form of fine white powders. The combined effects of P and N FRs in the modified styrenic polymers were examined, with P and N atoms within the same group (as in poly(S-co-ADEPMAE) and within different groups (as in poly(S-ter-DEAMP-ter-MI) and poly(S-ter-DEpVBP-ter-MI).
Structural characterisation of polymers
The chemical structures of the prepared polymers were confirmed by 1H NMR and FT-IR (ATR) spectroscopic techniques. From the 1H NMR spectra of the modified polymers, the degree of P and N incorporation and the molar concentration of monomeric units M1 and M2 were determined (Table 2).
The 1H NMR spectra of PS and modified polymers are provided as supplementary information (SI. 1). From the 1H NMR spectra the following characteristic peaks were identified at a chemical shift (δ) = 6.6 and 7.1 ppm, aromatic protons from styrene segments; methyl protons (O–CH2–CH3) from diethylphosphonate groups at δ = 1.1–1.2 ppm; methylene protons (O–CH2–CH3) from ethyl groups of different monomers at δ = 3.9 ppm; benzylic proton (Ph–CH2–P) from DEpVBP fragment at δ = 3.1 ppm. The common signals of small intensity observed at 2.3 ppm and 7.26 ppm originate from the traces of the solvent toluene.
The FT-IR (ATR) spectra of PS and corresponding co- and ter- polymers also confirmed the introduction of the monomers into the PS main chain (SI. 2). In addition to the characteristic peaks in the region from 3100 to 2850 cm−1, in the spectrum of PS (dotted areas), the spectra of co- and ter- polymers showed additional specific absorption peaks at 1730–1741 cm−1 (–C=O), 1250–1260 cm−1 (–P=O) and 1025 cm−1 (–P–O–C), confirming the presence of chemically bonded DEAMP, ADEPMAE, DEpVBP and MI groups in the polymer chains [9, 20, 21]. Moreover, the FT-IR spectra of ter-polymers showed the absorptions at 1340 cm−1 (C–N), indicating that the N-containing units are effectively incorporated into the polymeric chains.
Thermogravimetric analysis
The thermogravimetric analysis of P-, P-/N- and N-monomers was carried out under nitrogen and air atmospheres to evaluate the structural influence of the monomers on thermal behaviours of corresponding polymers. The comparison of TG traces of the monomers obtained under both atmospheres (Fig. 3) revealed that the P- containing monomer, DEpVBP, exhibited a different degradation pattern and had better char-forming ability than other monomers. The high residue content of DEpVBP may be a result of oligo- or poly-aromatic structures formation in the end of thermal decomposition process. Meanwhile, the aliphatic monomer (DEAMP) and the P–N monomer (ADEPMAE) were less thermally resistant. The lower thermal stability of N- containing monomer, MI, showed that nitrogen group alone cannot significantly alter thermal behaviours of polymeric chains.
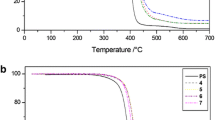
The thermal degradation of polymers was studied by TGA under both nitrogen and air atmospheres. Figure 4 presents the TGA and derivative thermogravimetric (DTG) curves of PS and styrenic polymers modified with corresponding FR groups under the nitrogen atmosphere. The summary of TGA data is given in Table 3.
In the inert atmosphere, PS undergoes a significant one-step degradation in the temperature range of 399–500 °C, which can be thought to arise from an initial phase of chain scissions (i.e., random, or chain-end, scissions), followed by the formation of styrene monomer, oligomers and some volatiles (Fig. 4 and Table 3) [22, 23]. However, the thermal degradation pattern of PS was substantially altered after the incorporation of either one type of FR group (i.e., in co-polymers), or two different types of FR groups (i.e., in ter-polymers). Most importantly, the co-polymers with DEAMP, DEpVBP and ADEPMAE monomeric units, exhibited two-step thermal degradation profiles, whereas the co-polymer with MI groups had only one step, which was quite similar to the one recorded for the unmodified PS (Fig. 4a). Indeed, the temperature corresponding to the initial mass loss in all co-polymers was lowered compared to the unmodified PS. This could be attributed to an early thermal cracking of the P- and N-containing groups, prior to the onset of the main chain decomposition of PS [6, 24]. The first degradation step, associated with 5.9–6.6% mass loss, for P- and P-/N- containing co-polymers was observed in the temperature range of 310–360 °C (Fig. 4a). This small step, registered before the main degradation step of PS, may be attributed to the release of ethylene molecules from the alkyl phosphonate moiety via cyclic-intermediate assisted reaction as previously reported [6, 11]. It can be noted from Fig. 4 that the mass loss rate of co-polymers was lower than that of PS. The decrease in the mass loss rate indicates that the covalently bonded monomeric units slow down, or alter, the pyrolytic route(s) of PS. As for the char yields, PS produced very little residue at 800 °C under nitrogen, whereas the degradation of co-polymers containing DEAMP, DEpVBP, ADEPMAE and MI units, resulted in the increased char residues (by about 2–6%) (Fig. 4 and Table 3). The char formation is an important factor that positively influences the thermal stability of polymers, through the condensed-phase mechanism. Generally, the produced char acts as a physical barrier, which can prevent both the heating of the unpyrolysed material and the associated release of combustible gases, thereby increasing the fire resistance of polymers [15]. Clearly, the reactive modification of PS with P- or P-/N- and N-groups can enhance the thermal behaviour of PS, especially, at elevated temperatures in an inert atmosphere [17].
The TGA results of styrenic co-polymers clearly demonstrated that the DEAMP, DEpVBP and MI units, once chemically incorporated into the polymeric chains of PS, altered its thermal degradation and char formation capabilities. Therefore, these monomeric units were selected for the further study, i.e., for the preparation of ter-polymers of styrene with a view to assessing the P–N cooperative effects, if any, exerted by these groups. Similar to the P- and P-/N- containing co-polymers, the thermal degradation of ter-polymers (poly(S-ter-DEAMP-ter-MI) and poly(S-ter-DEpVBP-ter-MI)) also exhibited a two-step degradation process (Fig. 4c, d). However, the degradation profile was somewhat different compared to that of co-polymers. For instance, in the case of poly(S-ter-DEAMP-ter-MI) the first mass loss started to occur at a temperature 178 °C lower than that of the corresponding co-polymer, poly(S-co-DEAMP). Meanwhile, for poly(S-ter-DEpVBP-ter-MI) the loss of mass commenced at a temperature 192 °C lower than that of the co-polymer, poly(S-co-DEpVBP). The earlier start of thermal degradation of ter-polymers could be explained by the initial breakdown of pendant P-groups as discussed earlier, which possibly could be influenced by the incorporated MI groups.
Another important finding is related to the char formation in the ter-polymers. It was observed that the char residue, obtained at 800 °C in nitrogen, increased by 44 times, from 0.5% for homo-polymer PS to 22.2% for poly(S-ter-DEAMP-ter-MI) and to 21.6% for poly(S-ter-DEpVBP-ter-MI) (Table 3, Fig. 4c). This significant increase in the amount of char generated by ter-polymers indicated a noticeable enhancement in the overall thermal stability of styrene-based polymers. It can be assumed that this behaviour is affected through cooperative interactions between the P- and N- containing units. It is also likely that the thermal decomposition of ter-polymers resulted in the formation of P–N intermediates, which promoted charring reactions, and reduced the overall rate of decomposition reactions, as revealed by the TGA and DTG curves (Fig. 4c, d) [25]. It is also interesting to note that P-N cooperative interactions appeared to be limited, if P and N atoms are contained within the same modifying group (ADEPMAE); i.e., a modest increase in charring (2%) was registered. Hence, it can be assumed that certain ‘neighbouring’ group participation was possible, when P and N atoms were in different pendent groups. In any case, the presence of phosphonate ester moieties are believed to promote the phosphorylation of phenyl groups through the in situ production of phosphoric acid [25]. This process may improve the retention of phosphorus in the condensed-phase, and hence promote char formation and its further stabilization [26].
The TGA and DTG curves of styrene-based polymers under the air atmosphere are presented in Fig. 5. The evaluated thermal parameters are detailed in Table 3. The TG data obtained in the air showed that the loss of mass in all the polymers started at temperatures 20–80 °C lower than those recorded under the inert atmosphere, (Fig. 5 and Table 3). This is expected as, generally, the presence of oxygen initiated an earlier thermal-oxidative degradation of polymers [11]. Nevertheless, under the air atmosphere, at 800 °C, the char forming ability of ter-polymers increased, from 0.4% for PS to 3.6% for poly(S-ter-DEAMP-ter-MI) and to 1.2% for poly(S-ter-DEpVBP-ter-MI). In the oxidative atmosphere, for the co- and ter-polymers, a third decomposition step was registered at the temperatures above 550 °C (Fig. 5), due to the secondary oxidation processes. The residues formed in the oxidative atmosphere at 500 °C by poly(S-co-DEAMP), poly(S-co-DEpVBP) and ter-polymers were 10–15% higher than those obtained in the inert atmosphere. Meanwhile, for the co-polymers, poly(S-co-MI) and poly(S-co-ADEPMAE), the mass residues at 500 °C were 6.9% and 9.2%, respectively. The solid residues underwent further oxidation as temperature increased up to 800 °C. For example, the ter-polymer, poly(S-ter-DEAMP-ter-MI) retained almost 44% of its initial mass at 500 °C, which then reduced rather rapidly to 3.6% at 800 °C. The char oxidation was also clearly visible from the DTG curves (Fig. 5d) in the 500–800 °C temperature interval, with Tmax = 594 °C for poly(S-ter-DEAMP-ter-MI) and Tmax = 600 °C for poly(S-ter-DEpVBP-ter-MI). In the oxidative atmosphere, the cooperative interactions between P- and N- bearing groups were evident from the thermal degradation behaviours of the co- and ter- polymers (poly(S-co-ADEPMAE), poly(S-ter-DEAMP-ter-MI) and poly(S-ter-DEpVBP-ter-MI)). The more pronounced effects were observed when polymer chains incorporated different P- and N- bearing moieties. Thus, it can be stated that the ter-polymers of styrene exhibited better overall thermal stability than homo- and co-polymers, in the oxidative atmosphere, showing excellent char-forming abilities, until the temperature of about 600 °C.
Differential scanning calorimetry
The DSC plots obtained for the co- and ter- polymers under the nitrogen atmosphere are presented in the supplementary part SI. 3. The neat PS underwent a single stage endothermic decomposition in the temperature range of 300–452 °C [27]. Meantime, the co-polymers poly(S-co-DEAMP) and poly(S-co-DEpVBP), as opposed to the unmodified PS, demonstrated an additional endothermic peak in the temperature range of 280–330 °C, with the endotherm being larger for the co-polymer containing DEAMP units. The initial endotherm correlates well with the degradation step registered on the corresponding TG curve. Meanwhile, an exotherm was observed at 264–300 °C on the DSC curves of poly(S-co-ADEPMAE). This could be associated with the release (and possible interaction) of ethylene from the ethyl groups of the ‘side arms’ of the phosphonate groups. The larger endotherm, in the temperature interval from 350 to 470 °C for all the co-polymers, can be attributed to the main chain decomposition step, also shown on the TG curves recorded under the nitrogen atmosphere [11]. It can also be observed that reactively modified polymers exhibited the lower heat of pyrolysis (∆Hpyro) than that of the unmodified PS (∆Hpyro of PS and modified styrenic polymers are provided in SI. 4). Among the co-polymers, the lowest value of ∆Hpyro was observed for poly(S-co-DEAMP), 393 J/g, nearly a half of the value found for PS. Furthermore, the ∆Hpyro value of ter-polymers decreased dramatically from 717 J/g for PS to 191 J/g, and 217 J/g for poly(S-ter-DEAMP-ter-MI) and poly(S-ter-DEpVBP-ter-MI), respectively. The decrease in ∆Hpyro may be influenced by several factors, such as the difference in the energy requirements for the bond cleavage of the polymeric chains and thermal energy requirements for the initial cracking of pendant P- and N- groups. In the modified styrenic polymers, an earlier thermal cracking and enhanced char production, especially, after the degradation of the main chain, possibly resulted in the reduced production of volatiles, leading to an overall decrease in values of heat of pyrolysis. The fragments that bear P and/or N atoms, which are assumed to be produced during the earlier stages of the decomposition of the modified polymeric systems, are believed to exert combustion inhibition in the gaseous phase.
Combustion properties of polymers
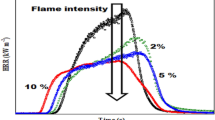
The combustion properties of PS and styrene-based co- and ter- polymers were studied using PCFC, and the detailed data of measured parameters are given in Table 4. Among various combustion parameters, the HRC serves as a reliable indicator of a polymer’s flammability [17, 28]. The curves of HRR as functions of temperature are shown in Fig. 6 for all polymers. Evidently, the incorporation of P- monomeric units into the PS chain resulted in a decrease of pHRR. As shown in Table 4, the pHRR of the neat PS was 752 W/g; it was reduced by almost 14% for the poly(S- co-DEAMP). In contrast, the pHRR of poly(S-co-MI) was increased to 790 W/g. The results demonstrated that the incorporation of N- groups alone into the polymeric chains cannot significantly alter the combustibility of PS, which is in agreement with the results obtained from TGA and DSC. However, the presence of nitrogen could improve the efficiency of P- containing FRs via P–N cooperative effect [25]. For instance, among the co-polymers, poly(S-co-ADEPMAE) had the lowest HRR value compared to the unmodified PS. The modification of PS chains with ADEPMAE units reduced the HRR by almost 22%. A similar trend was observed for the measured HRC values of poly(S-co-ADEPMAE). This reduction in the pHRR and HRC registered in P- containing co-polymers can be attributed to the release of non-flammable gases such as CO2, H2O, phosphorus compounds, etc. which could dilute the mixture of flammable pyrolysis products generated by thermal decomposition of PS [16]. Indeed, the small HRR peak at 306–370 °C, observed in Fig. 6 for the co-polymers with DEAMP, DEpVBP and ADEPMAE units, correlated well with the small shoulder visible on the DTG curves of the corresponding co-polymers (Fig. 4b).
Furthermore, the ter-polymers with P- and N- groups exhibited almost a 50% reduction in pHRR (55% for poly(S-ter-DEAMP-ter-MI) and 47% for poly(S-ter-DEpVBP-ter-MI)) compared to that of PS. The HRC values of both ter-polymers were also reduced in a similar pattern. This significant reduction of pHRR and HRC values is highly relevant in the case of polymers such as PS, especially in dictating their inherent fire hazards. Along with the number of non-flammable gases mentioned earlier, the presence of both P- and N-groups in the polymeric chains of ter-polymers may trigger the release of other products such as ammonia (NH3) or nitrogen (N2), which can further dilute the flammable mixture of volatiles [14]. The total amount of heat released, THR, can be used to assess the size of a fire and its ensuing fire hazards. The reactive modification of PS with P- and both P-/N-groups also resulted in the decrease of THR values. The lower values of pHRR and THR can be explained by the higher char formation (via P–N cooperative effects) during the thermal degradation of the modified polymers, which consequently reduced the generation of combustible fuels, and restrained the flame spread [13, 16]. Thus, the findings agree that the higher the char residues produced by ter-polymers, the lower the measured values of pHRR, HRC and THR (Table 4).
Another important parameter is the value of EHC, which depends on the heat of combustion of the volatile species generated from the degradation materials [15, 29]. Compared to the PS, the EHC values for the co-polymers with DEAMP, ADEPMAE and MI units were slightly reduced, whereas for the poly(S-co-DEpVBP) the EHC was increased (Table 4). The lower EHC may be associated with the gaseous-phase fire retarding effect of P-containing FRs during combustion [15]. For the ter-polymers, the EHC values were reduced by 29% for poly(S-ter-DEAMP-ter-MI) and 18% for poly(S-ter-DEpVBP-ter-MI), which indicated that the combustion inhibitory efficiency of P-FRs was enhanced by the presence of N-containing groups. The PCFC results revealed that reactive modification of PS with P- and N-moieties exerts combustion inhibition and enhances thermal behaviour of the polymer by improving the char formation (condensed-phase mechanism) and also through the gaseous-phase mode of action.
The heat of combustion (∆Hcomb) values of the modified and unmodified PS are presented in Table 4. The ∆Hcomb of all the modified polymers, as compared to the neat PS, was reduced due to the combustion inhibitory effects of the incorporated groups. Among the co-polymers, the ∆Hcomb was decreased by 16% for poly(S-co-MI), by 12% for poly(S-co-DEAMP) and by 6% for poly(S-co-ADEPMAE). However, for the co-polymer with DEpVBP groups the influence on ∆Hcomb value was found to be negligible. The observed reduction in ∆Hcomb values of the co-polymers may indicate that the incorporated monomeric units produced volatiles upon decomposition, which, in turn, exerted some degree of combustion inhibition. It can be assumed that the co-polymer with N- monomeric unit (poly(S-co-MI)) had a greater gaseous-phase inhibitory effect compared to other modified co-polymers.
The ter-polymerisation of styrene with different P- and N-monomers resulted in much lower values of ∆Hcomb than that of the co-polymers. For the poly(S-ter-DEAMP-ter-MI), the ∆Hcomb value was reduced by 23%, while for the poly(S-ter-DEpVBP-ter-MI) by almost 20%. The reduction in ∆Hcomb of ter-polymers can be attributed to the cooperative action of nitrogen within MI monomeric units and phosphorus within DEAMP and DEpVBP units. It is highly relevant to note that P–N cooperative influence was more noticeable in the polymers when P and N atoms were positioned within different modifying units than that in the polymer containing P and N within the same group (i.e., in ADEPMAE). This finding is in agreement with the TGA and PCFC results discussed earlier, indicating some sort of ‘neighbouring’ group effect.
A comparison of the P–N cooperative influence
The results presented in previous sections clearly indicated that there is an interaction (physical and/or chemical) between the PS matrix and the modifying FR groups. It is known that many N-containing compounds, despite having limited fire retardance themselves, can improve the fire retarding efficiency of P-containing FRs via P–N cooperative effects [26]. From the thermal and calorimetric testing results detailed in the current study, it is obvious that combustion inhibition of PS has been significantly increased by the incorporation of P- and N-containing groups, possibly by exerting some cooperative effects. However, it is highly desirable to evaluate the degree of fire retardance enhancement, and the dependence of a P–N influence on different chemical environments of P and N atoms. This was done by the authors by making some generalisations of relevant parameters obtained from TGA (at 60 °C min−1, under the nitrogen atmosphere, TG curves are provided in the supplementary section SI. 5), PCFC, and ‘bomb’ calorimetry. It is also relevant to note that the loadings (mass %) of P or N in the modified systems were comparable (P ~ 3–5%, and N ~ 3–4%).
The plots of char residue formed in TGA (60 °C min−1) and PCFC tests, are presented in Fig. 7a for PS and modified styrenic polymers. It can be noted that the neat PS yielded a near zero char residue in both tests. However, the char residue was increased following the modification of PS with different P- and N-groups, indicating the effect of P–N cooperative actions. The polymer containing group with P–N bonds (in poly(S-co-ADEPMAE)) showed a modest increase of the char yield to 2.5% in TGA, while in PCFC test, char yield remained 0%. Meanwhile, for the polymers with different P- and N-moieties the char yields were significantly increased in both tests. For example, poly(S-ter-DEAMP-ter-MI) demonstrated an increase of 25% (TGA) and 16% (PCFC), while for poly(S-ter-DEpVBP-ter-MI) char yield increased to 18% (TGA) and 20% (PCFC). The enhanced char yield is potentially linked to the cooperative action of nitrogen and phosphorus, resulting in the formation of various P–N intermediates. It can be assumed that this process may retain phosphorus in the condensed-phase, thereby making the polymer more thermally stable. The results indicated that the incorporation of P (e.g., DEAMP or DEpVBP) and N atoms (MI) within different modifying units renders the polymer more thermally resistant attributes compared to the polymer containing P and N atoms within the same group, as in poly(S-co-ADEPMAE).
As it can be seen from Fig. 7b, the ΔHcomb values obtained from ‘bomb’ calorimetry (i.e., in the case of complete combustion) were higher than the corresponding EHC values calculated from PCFC (i.e., the case of incomplete and forced non-flaming combustion) for all the polymers. Figure 7b also reveals that the chemical environments of P and N atoms influenced the cooperative action. In comparison with the poly(S-co-ADEPMAE), other two polymers such as poly(S-ter-DEAMP-ter-MI) and poly(S-ter-DEpVBP-ter-MI) lowered the ΔHcomb by 18% and 14%, respectively, while the EHC was reduced by 28% and 17%, respectively. Thus, the values of EHC and ΔHcomb, which are primarily related to the gaseous-phase activity of a FR, revealed that the P–N cooperative effects were more pronounced in the polymers where P and N were present in separate modifying groups. From the above-mentioned analysis, it can be concluded that chemical and physical interactions occurring during thermal degradation of the modified polymers improved the combustion inhibition through a combination of the condensed- and gaseous-phase activities.
In addition, it is equally important to compare the chemical environment of P atoms within the modifying groups bonded to the main chain. In the case of poly(S-ter-DEAMP-ter-MI), P atom has an aliphatic surrounding within the DEAMP group, while in the case of poly(S-ter-DEpVBP-ter-MI), P atom is within the aromatic moiety, DEpVBP. The thermogravimetric studies of these monomers (Fig. 3) demonstrated that the aromatic monomer DEpVBP had better thermal stability than the aliphatic monomer DEAMP. However, thermal behaviours and combustion characteristics of ter-polymers showed that the polymer with the aliphatic P-moiety (poly(S-ter-DEAMP-ter-MI)) had performed better at elevated temperatures compared to the one with aromatic P-moiety. This is in the agreement with the results obtained for the corresponding co-polymers with DEAMP and DEpVBP units. The P content calculated from 1H NMR spectra was higher for poly(S-co-DEAMP) than for poly(S-co-DEpVBP): 3.07 and 1.87 mass %, respectively. The higher incorporation of DEAMP units, as compared to DEpVBP, could be explained by the fact that DEAMP is an acceptor-type monomer, and hence would tend to copolymerise more easily with styrene, which is considered as a donor type monomer [12]. The same trend was observed in the case of ter-polymers (see Table 2), poly(S-ter-DEAMP-ter-MI) had a higher P content than the ter-polymer containing DEpVBP monomeric units. However, it is also important to note that despite a lower P content, poly(S-ter-DEpVBP-ter-MI) performed better in terms of char formation and other combustibility parameters. This may be due to the presence of aromatic rings within the P-containing group, DEpVBP, which tend to form char precursors upon heating.
Conclusions
In this study, three different P-containing unsaturated compounds (DEAMP, DEpVBP and ADEPMAE) and one N-containing compound, MI, were used for the preparation of styrene-based co-and ter-polymers. Thermal and combustibility characterisation showed that the co-polymer with incorporated DEAMP units had better performance compared to poly(S-co-DEpVBP) and poly(S-co-ADEPMAE). From thermal and calorimetric analyses, it was found that the presence of only nitrogen within a FR group did not significantly alter the thermal and combustion properties of PS. For the first time, the synthesis and characterisation of ter-polymers of styrene, with different functional monomers (DEAMP, DEpVBP and MI), were carried out in the present work. The TGA results of the ter-polymers indicated the influence of cooperative interactions between P- and N-containing groups on the thermal degradation patterns and combustion attributes of styrene-based polymers. It was established that chemical incorporation of P- and N-groups resulted in an overall increase of thermal stabilities, in a lower mass loss rate and a higher char formation (e.g., above 20% at 800 °C, under the nitrogen atmosphere) of the modified polymeric products. More importantly, the ter-polymers demonstrated almost a 50% reduction in the pHRR and HRC values. In addition, the ter-polymers displayed the lower heats of combustion as opposed to the neat PS. A comparison of thermal and calorimetric characteristics of the modified styrenic polymers also revealed that the extent of the cooperative interactions between P and N strongly depended on the chemical environments and binding patterns of P and N atoms. The incorporation of P and N into the polymeric chains as separate DEAMP, DEpVBP and MI units made these polymers more resilient to thermal degradation compared to a polymer containing P and N atoms within the same pendent group (i.e., in the case of poly(S-co-ADEPMAE)). The results from various tests also pointed towards both condensed- and gaseous-phase activities of FR groups. The physio-chemical processes dictating the exact mode of action of the modifying groups will be published separately.
References
Lynwood C. Polystyrene: synthesis, characteristics, and applications. New York: Nova Science Publishers; 2014.
Weil ED, Levchik SV. Flame retardants for polystyrenes in commercial use or development. J Fire Sci. 2007;25:241–65.
Ezechiá M, Covino S, Cajthaml T. Ecotoxicity and biodegradability of new brominated flame retardants: a review. Ecotoxicol Environ Saf. 2014;110:153–67.
Joseph P, Tretsiakova-Mcnally S. Reactive modifications of some chain- and step-growth polymers with phosphorus-containing compounds: effects on flame retardance—a review. Polym Adv Technol. 2011;22:395–406.
Baby A, Tretsiakova-McNally S, Arun M, Joseph P, Zhang J. Reactive and additive modifications of styrenic polymers with phosphorus-containing compounds and their effects on fire retardance. Molecules. 2020;25:1–36.
Dumitrascu A, Howell BA. Flame-retarding vinyl polymers using phosphorus-functionalized styrene monomers. Polym Degrad Stab. 2011;96:342–9.
Yan L, Zheng YB, Liang X, Ma Q. Copolymerization of 1-Oxo-2,6,7-trioxa-1-phorsphabicyclo [2,2,2]oct-4-yl methyl acrylate and (10-Oxo-10-Hydro-9-Oxa-10-phosphaphenanthrene-10-yl) methyl acrylate with styrene and their thermal degradation characteristics. J Appl Polym Sci. 2010;115:1032–8.
Howell BA, Daniel YG. Incorporation of comonomer exo-5-(Diphenylphosphato)Isosorbide-2-endo-acrylate to generate flame retardant poly(styrene). Polym (Basel). 2019;11:2038.
Dumitrascu A, Howell BA. Flame retardant polymeric materials achieved by incorporation of styrene monomers containing both nitrogen and phosphorus. Polym Degrad Stab. 2012;97:2611–8.
Canadell J, Hunt BJ, Cook AG, Mantecón A, Cádiz V. Flame retardance and shrinkage reduction of polystyrene modified with acrylate-containing phosphorus and crosslinkable spiro-orthoester moieties. Polym Degrad Stab. 2007;92:1482–90.
Price D, Cunliffe LK, Bullett KJ, Hull TR, Milnes GJ, Ebdon JR, et al. Thermal behaviour of covalently bonded phosphate and phosphonate flame retardant polystyrene systems. Polym Degrad Stab. 2007;92:1101–14.
Ebdon JR, Price D, Hunt BJ, Joseph P, Gao F, Milnes GJ, et al. Flame retardance in some polystyrenes and poly(methyl methacrylate)s with covalently bound phosphorus-containing groups: initial screening experiments and some laser pyrolysis mechanistic studies. Polym Degrad Stab. 2000;69:267–77.
Tai Q, Song L, Hu Y, Yuen RKK, Feng H, Tao Y. Novel styrene polymers functionalized with phosphorus-nitrogen containing molecules: synthesis and properties. Mater Chem Phys. 2012;134:163–9.
Tai Q, Chen L, Song L, Nie S, Hu Y, Yuen RKK. Preparation and thermal properties of a novel flame retardant copolymer. Polym Degrad Stab. 2010;95:830–6.
Cui J, Zhu C, He M, Ke Z, Liu Y, Tai Q, et al. Preparation and thermal properties of a novel core-shell structure flame-retardant copolymer. Polym Adv Technol. 2018;29:541–50.
Hu W, Zhan J, Hong N, Hull TR, Stec AA, Song L, et al. Flame retardant polystyrene copolymers: preparation, thermal properties, and fire toxicities. Polym Adv Technol. 2014;25:631–7.
Tretsiakova-McNally S, Joseph P. Pyrolysis combustion flow calorimetry studies on some reactively modified polymers. Polym (Basel). 2015;7:453–67.
Tretsiakova-McNally S, Joseph P. Thermal and calorimetric evaluations of polyacrylonitrile containing covalently-bound phosphonate groups. Polym (Basel). 2018;10:131:1-131:14.
John R. Ebdon, Barry J. Hunt, Paul Joseph, Tara. K. Wilkie. Flame retardance of polyacrylonitriles covalently modified with phosphorus- and nitrogen- containing groups. In: Hull TR, Kandola BK, editors. Fire Retard Polymers in the New Strategy Mechics. Royal Society of Chemistry, Cambridge, UK; 2009. pp. 331–340.
Olmos D, Martín EV, González-Benito J. New molecular-scale information on polystyrene dynamics in PS and PS-BaTiO3 composites from FTIR spectroscopy. Phys Chem Chem Phys. 2014;16:24339–49.
Ramesh S, Sivasamy A, Kim J. Synthesis and characterization of maleimide- nanocomposites by Sol-Gel process. Nanoscale Res Lett. 2012;7:350–63.
Faravelli T, Pinciroli M, Pisano F, Bozzano G, Dente M, Ranzi E. Thermal degradation of polystyrene. J Anal Appl Pyrolysis. 2001;60:103–21.
Bob A. H. The mechanism of poly(styrene) degradation. In: Tiwari A, Raj B, editors. Reaction in the Mechnical and Thermal Analysis in the Advances Materials. 2015; pp. 259–266.
Joseph P, Tretsiakova-McNally S. Combustion behaviours of chemically modified polyacrylonitrile polymers containing phosphorylamino groups. Polym Degrad Stab. 2012;97:2531–5.
Gaan S, Sun G, Hutches K, Engelhard MH. Effect of nitrogen additives on flame retardant action of tributyl phosphate: phosphorus-nitrogen synergism. Polym Degrad Stab. 2008;93:99–108.
Markwart JC, Battig A, Zimmermann L, Wagner M, Fischer J, Schartel B, et al. Systematically controlled decomposition mechanism in phosphorus flame retardants by precise molecular architecture: P-O vs P–N. ACS Appl Polym Mater Am Chem Soc. 2019;1:1118–28.
Petrella RV. Factors affecting the combustion of polystyrene and styrene. In: Lewin M, Atlas SM, Pearce EM, editors. Flame-Retardant Polymer in Materials. Plenum Press: New York; 1978. p. 159–201.
Babrauskas V, Peacock RD. Heat release rate: the single most important variable in fire hazard. Fire Saf J. 1992;18:255–72.
Schartel B, Hull TR. Development of fire-retarded materials-interpretation of cone calorimeter data. Fire Mater. 2007;31:327–54.
Acknowledgements
AB is grateful for the provision of the overseas PhD studentship from Ulster University, UK
Funding
This research was supported by the Royal Society of Chemistry (ID R19-3521).
Author information
Authors and Affiliations
Contributions
AB led the preparation of the manuscript. AB structured the manuscript and drafted the main text. AB carried out all the synthetic work. AB, PJ and MA carried out characterisation of the obtained polymers. AB, STMc, JZ, PJ and DP carried out interpretation of the results. STMc and PJ conceptualized the research. STMc and JZ supervised the research. AB, STMc, PJ, MA, JZ and DP contributed to the manuscript by writing and reviewing different sections of the article. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baby, A., Tretsiakova-McNally, S., Joseph, P. et al. The influence of phosphorus- and nitrogen- containing groups on the thermal stability and combustion characteristics of styrenic polymers. J Therm Anal Calorim 148, 229–241 (2023). https://doi.org/10.1007/s10973-022-11404-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11404-6