Abstract
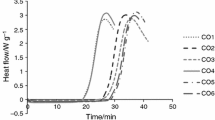
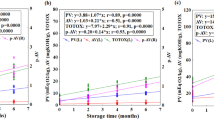
There is growing interest worldwide in the use of camelina oil for food as well as for biofuel purposes. For both of these applications, oxidative stability is an important feature of the oil. Therefore, the aim of this study was to test the thermal resistance to oxidation of three different cultivars of camelina oil i.e., Omega, Luna and Śmiłowska by means of isothermal and non-isothermal differential scanning calorimetry (DSC) oxidation measurement. For isothermal DSC analysis, different temperatures were tested (120, 140, 160 °C) and in the non-isothermal mode different scanning rates (1, 2, 5, 10, 15 °C min−1) were used. To support the DSC data, chemical analyzes were also performed i.e., fatty acid composition, peroxide value, p-anisidine value, acid value and radical scavenging activity by 2,2-diphenyl-1-picrylhydrazyl (RSA DPPH). The isothermal test indicated that for all camelina oils the oxidation induction time (OIT) decreased with an increase in temperature on average from 69.83 min for 120 °C to 5.13 min for 160 °C. The OIT values corresponded very well with non-isothermal DSC results, for which the onset temperatures (Ton) increased with the increase of heating rate on average from 142.15 °C for 1 °C min−1 to 185.75 °C for 15 °C min−1. The parameters of DSC oxidative stability i.e., OIT as well as Ton values were negatively correlated with some unsaturated fatty acids content e.g., α-linolenic acid (C18:3, n-3) and positively with yellowness b* and RSA DPPH. Oil from camelina seeds of Śmiłowska cultivar, which was characterized by the lowest content of α-linolenic acid and the highest b* value of color and RSA DPPH, was the most thermally stable oil.








Similar content being viewed by others
References
Budin JT, Breene WM, Putnam DH. Some compositional properties of camelina (camelina sativa L. Crantz) seeds and oils. J Am Oil Chem Soc. 1995;72:309–15.
Crowley JG, Fröhlich A. (1998) Factors affecting the composition and use of Camelina. End Proj Reports, Teagasc. 1–23
Ibrahim FM, El Habbasha SF. Chemical composition, medicinal impacts and cultivation of camelina (Camelina sativa): review. Int J PharmTech Res. 2015;8:114–22.
Abramovič H, Abram V. Physico-chemical properties, composition and oxidative stability of camelina sativa oil. Food Technol Biotechnol. 2005;43:63–70.
Ratusz K, Popis E, Ciemniewska-Żytkiewicz H, Wroniak M. Oxidative stability of camelina (Camelina sativa L.) oil using pressure differential scanning calorimetry and Rancimat method. J Therm Anal Calorim. 2016;126:343–51.
Ratusz K, Symoniuk E, Wroniak M, Rudzińska M. Bioactive compounds, nutritional quality and oxidative stability of cold-pressed camelina (Camelina sativa L.) oils. Appl Sci. 2018;8:1–17.
Piravi-vanak Z, Azadmard-Damirchi S, Kahrizi D, Mooraki N, Ercisli S, Savage GP, et al. Physicochemical properties of oil extracted from camelina (Camelina sativa) seeds as a new source of vegetable oil in different regions of Iran. J Mol Liq. 2021;345:117043.
Gunstone FD, Morris LJ. Fatty acids. Part VI. The oxygenated acid present in Camelina sativa (Crantz.) seed oil. J Chem Soc. 1959. https://doi.org/10.1039/jr9590002127.
Abramovič H, Butinar B, Nikolič V. Changes occurring in phenolic content, tocopherol composition and oxidative stability of Camelina sativa oil during storage. Food Chem. 2007;104:903–9.
Berti M, Gesch R, Eynck C, Anderson J, Cermak S. Camelina uses, genetics, genomics, production, and management. Ind Crops Prod. 2016;94:690–710.
Zubr J, Matthaus B. Effects of growth conditions on fatty acids and tocopherols in Camelina sativa oil. Ind Crops Prod. 2002;15:155–62.
Gugel RK, Falk KC. Agronomic and seed quality evaluation of Camelina sativa in western Canada. Can J Plant Sci. 2006;86:1047–58.
Kurasiak-Popowska D, Graczyk M, Przybylska-Balcerek A, Stuper-Szablewska K. Influence of variety and weather conditions on fatty acid composition of winter and spring Camelina sativa varieties in Poland. Eur Food Res Technol. 2021;247:465–73. https://doi.org/10.1007/s00217-020-03639-0.
Moslavac T, Jokić S, Šubarić D, Aladić K, Vukoja J, Prce N. Pressing and supercritical CO2 extraction of Camelina sativa oil. Ind Crops Prod. 2014;54:122–9.
Kiralan M, Kiralan SS, Subaşi I, Aslan Y, Ramadan MF. Fatty acids profile and stability of camelina (Camelina sativa) seed oil as affected by extraction method and thermal oxidation. Riv Ital delle Sostanze Grasse. 2018;95:223–8.
Urbaniak SD, Caldwell CD, Zheljazkov VD, Lada R, Luan L. The effect of seeding rate, seeding date and seeder type on the performance of Camelina sativa L. in the Maritime Provinces of Canada. Can J Plant Sci. 2008;88:501–8.
Ciubota-Rosie C, Ruiz JR, Ramos MJ, Pérez Á. Biodiesel from Camelina sativa: a comprehensive characterisation. Fuel Elsevier Ltd. 2013;105:572–7.
Yang J, Caldwell C, Corscadden K, He QS, Li J. An evaluation of biodiesel production from Camelina sativa grown in Nova Scotia. Ind Crops Prod. 2016;81:162–8.
Obeng E, Obour AK, Nelson NO, Moreno JA, Ciampitti IA, Wang D, et al. Seed yield and oil quality as affected by Camelina cultivar and planting date. J Crop Improv Taylor Francis. 2019;33:202–22.
Belayneh HD, Wehling RL, Cahoon EB, Ciftci ON. Effect of extraction method on the oxidative stability of camelina seed oil studied by differential scanning calorimetry. J Food Sci. 2017;82:632–7.
Belayneh HD, Wehling RL, Cahoon E, Ciftci ON. Extraction of omega-3-rich oil from Camelina sativa seed using supercritical carbon dioxide. J Supercrit Fluids. 2015;104:153–9.
Szterk A, Roszko M, Sosińska E, Derewiaka D, Lewicki PP. Chemical composition and oxidative stability of selected plant oils. JAOCS, J Am Oil Chem Soc. 2010;87:637–45.
Raczyk M, Popis E, Kruszewski B, Ratusz K, Rudzińska M. Physicochemical quality and oxidative stability of linseed (Linum usitatissimum) and camelina (camelina sativa) cold-pressed oils from retail outlets. Eur J Lipid Sci Technol. 2016;118:834–9.
Symoniuk E, Ratusz K, Ostrowska-Ligęza E, Krygier K. Impact of selected chemical characteristics of cold-pressed oils on their oxidative stability determined using the Rancimat and pressure differential scanning calorimetry method. Food Anal Methods. 2018;11:1095–104.
Tamási K, Marossy K. Combined thermal analysis of plant oils. J Therm Anal Calorim. 2021;147:2047–54. https://doi.org/10.1007/s10973-020-10470-y.
Eidhin DN, Burke J, O’Beirne D. Oxidative stability of ω3-rich Camelina oil and camelina oil-based spread compared with plant and fish oils and sunflower spread. J Food Sci. 2003;68:345–53.
Eidhin DN, O’Beirne D. Oxidative stability of camelina oil in salad dressings, mayonnaises and during frying. Int J Food Sci Technol. 2010;45:444–52.
Różańska MB, Kowalczewski PŁ, Tomaszewska-Gras J, Dwiecki K, Mildner-Szkudlarz S. Seed-roasting process affects oxidative stability of cold-pressed oils. Antioxidants. 2019;8:313. https://doi.org/10.3390/antiox8080313.
Hrastar R, Abramovič H, Košir IJ. In situ quality evaluation of Camelina sativa landrace. Eur J Lipid Sci Technol. 2012;114:343–51.
Kurasiak-Popowska D, Rynska B, Stuper-Szablewska K. Analysis of distribution of selected bioactive compounds in camelina sativa from seeds to pomace and oil. Agronomy. 2019;9:168.
Codex Alimentarius. (1999) Standard for named vegetable oils codex Stan 210–1999. Codex Aliment. 1–13.
Tomaszewska-Gras J, Islam M, Grzeca L, Kaczmarek A, Fornal E. Comprehensive thermal characteristics of different cultivars of flaxseed oil (Linum usittatissimum L.). Molecules. 2021;26:1–20.
Adhvaryu A, Erhan SZ, Liu ZS, Perez JM. Oxidation kinetic studies of oils derived from unmodified and genetically modified vegetables using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim Acta. 2000;364:87–97.
Ostrowska-Ligeza E, Bekas W, Kowalska D, Lobacz M, Wroniak M, Kowalski B. Kinetics of commercial olive oil oxidation: dynamic differential scanning calorimetry and Rancimat studies. Eur J Lipid Sci Technol. 2010;112:268–74.
Tan CP, Che Man YB, Selamat J, Yusoff MSA. Application of Arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. JAOCS, J Am Oil Chem Soc. 2001;78:1133–8.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B Polym Lett. 1966;4:323–8.
Qi B, Zhang Q, Sui X, Wang Z, Li Y, Jiang L. Differential scanning calorimetry study - Assessing the influence of composition of vegetable oils on oxidation. Food Chem. 2016;194:601–7. https://doi.org/10.1016/j.foodchem.2015.07.148.
Saldana MDA, Martinez-Monteagudo SI. (2013) Oxidative Stability of Fats and Oils Measured by Differential Scanning Calorimetry for Food and Industrial Applications. In: Amal Ali Elkordy, (Eds) Appl Calorim a Wide Context - Differ Scanning Calorimetry, Isothermal Titration Calorim. Microcalorim. IntechOpen https://doi.org/10.5772/54486
Itle RA, Kabelka EA. Correlation between lab color space values and carotenoid content in Pumpkins and Squash (Cucurbita spp.). HortScience. 2009;44:633–7.
Acknowledgements
Part of the results published in this article was presented at the conference “7th International Congress on Thermal Analysis and Calorimetry; 8th Joint Czech-Hungarian-Polish-Slovakian Thermoanalytical Conference; 14th Conference on Calorimetry and Thermal Analysis of the Polish Society of Calorimetry and Thermal Analysis” on August 29– September 2, 2021.
Funding
This research was funded by the NATIONAL SCIENCE CENTRE, POLAND, grant number: 2018/31/B/NZ9/02762.
Author information
Authors and Affiliations
Contributions
Conceptualization: Mahbuba Islam, Jolanta Tomaszewska-Gras; methodology Mahbuba Islam, Jolanta Tomaszewska-Gras, Małgorzata Muzolf-Panek; formal analysis: Mahbuba Islam, Jolanta Tomaszewska-Gras, Małgorzata Muzolf-Panek; investigation: Mahbuba Islam, Jolanta Tomaszewska-Gras, Małgorzata Muzolf-Panek; resources: Jolanta Tomaszewska-Gras; data curation: Mahbuba Islam, Jolanta Tomaszewska-Gras; writing—original draft preparation: Mahbuba Islam, Jolanta Tomaszewska-Gras; writing—review and editing: Mahbuba Islam, Jolanta Tomaszewska-Gras, visualization, Mahbuba Islam, Jolanta Tomaszewska-Gras; supervision: Jolanta Tomaszewska-Gras, project administration: Emilia Fornal, Jolanta Tomaszewska-Gras, funding acquisition: Emilia Fornal, Jolanta Tomaszewska-Gras. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Islam, M., Muzolf-Panek, M., Fornal, E. et al. DSC isothermal and non-isothermal assessment of thermo-oxidative stability of different cultivars of Camelina sativa L. seed oils. J Therm Anal Calorim 147, 10013–10026 (2022). https://doi.org/10.1007/s10973-022-11367-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11367-8