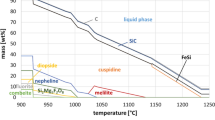
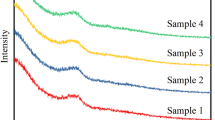
Abstract
In this study, a hot stage microscope, thermogravimetry–differential scanning calorimetry (TG-DSC), and a scanning electron microscope were used to study the role of carbonaceous materials in mold powder and their influence on the melting process. It was found that carbon black has a good isolation effect in mold powder. It delayed the solid-state reaction between the raw materials and increased the softening and hemispherical temperatures of the mold powder. Moreover, the steric hindrance effect of carbon black led to the formation of irregular 2CaO·SiO2 crystals in the solid-state reaction and a significant increase in the flow temperature that exceeded the 30 ℃ error limit of the current standard. The TG-DSC thermal analysis shows that during the heating process of mold powder with 15% graphite, the exothermic combustion of graphite accelerated the decomposition reaction of Na2CO3 and Li2CO3, promoted the melting of mold powder, and decreased the softening and hemispherical temperatures. However, the decrease was smaller than 30 ℃, and there was no need to decarbonize the carbon before the melting range test.












Similar content being viewed by others
Availability of data and material
Not acceptable.
Code availability
Not acceptable.
References
Mills KC. A short history of mould powders. Ironmak Steelmak. 2017;44:326–32. https://doi.org/10.1080/03019233.2017.1288367.
Mills KC, Däcker C-Å. The casting powders book. Cham: Springer International Publishing; 2017. https://doi.org/10.1007/978-3-319-53616-3
Singh D, Bhadwaj P, Yang YD, McLean A, Hasegawa M, Iwase M. The influence of carbonaceous material on the melting behavior of mould powder. Steel Res Int. 2010;81:974–9. https://doi.org/10.1002/srin.201000131.
Wei E, Yang Y, Feng C, Sommerville ID, McLean A. Effect of carbon properties on melting behavior of mold fluxes for continuous casting of steel. J Iron Steel Res Int. 2006;13:22–6. https://doi.org/10.1016/S1006-706X(06)60037-X.
Benavidez E, Santini L, Brandaleze E. Decomposition kinetic of carbonaceous materials used in a mold flux design. J Therm Anal Calorim. 2011;103:485–93. https://doi.org/10.1007/s10973-010-1081-5.
Kromhout J, Kamperman A, Kick M, Trouw J. Mould powders selection for thin slab casting. Ironmak Steelmak. 2005;32:127–32. https://doi.org/10.1179/174328105X15869.
Kargul T. The analysis of gas evolved from casting powder during heating in the mold. J Therm Anal Calorim. 2020;139:877–84. https://doi.org/10.1007/s10973-019-08468-2.
Kim J, Lee Y, Lee H. Decomposition of Li2CO3 by interaction with SiO2 in mold flux of steel continuous casting. ISIJ Int. 2004;44:334–41. https://doi.org/10.2355/isijinternational.44.334.
Marschall I, Kolbl N, Harmuth H, Atzenhofer C. Identification of secondary raw materials in mold powders and their melting behavior. J Iron Steel Res Int. 2019;26:403–11. https://doi.org/10.1007/s42243-019-00254-6.
Benavidez E, Santini L, Martin A, Brandaleze E. Master decomposition curve of carbonaceous materials used in casting powders. J Therm Anal Calorim. 2017;133:695–701. https://doi.org/10.1007/s10973-017-6892-1.
Brandaleze E, Valentini M, Santini L, Benavidez E. Study on fluoride evaporation from casting powders. J Therm Anal Calorim. 2017;133:271–7. https://doi.org/10.1007/s10973-018-7227-6.
YB/T 186–2014, Method of the test for melting temperature of continuous casting mould powder, 2014 (in Chinese).
DIN 51730: 2007, Testing of solid fuels-Determination of fusibility of fuel ash, 2007 (in German).
ISO 540: 2008, Hard coal and coke-Determination of ash fusibility, 2008.
D1857/D1857M: 2018, Standard test method for fusibility of coal and coke ash, 2018.
Hariharan A, Mozundar SK. Evolution of mould fluxes. Adv Mater Res. 2013;794:75–89. https://doi.org/10.4028/www.scientific.net/AMR.794.75.
Wang Q, Lu Y, He S, Wang L, Mills KC. Development of test method for measuring sintering temperature of mould fluxes. J Iron Steel Res Int. 2011;18:1–6. https://doi.org/10.1016/S1006-706X(11)60041-1.
Soares RW, Fonseca MVA, Neuman R, Menezes VJ, Lavinas AO, Dweck J. An application of differential thermal analysis to determine the change in thermal properties of mold powders used in continuous casting of steel slab. Thermochim Acta. 1998;318:131–6. https://doi.org/10.1016/S0040-6031(98)00337-2.
Nakada H, Fukuyama H, Nagata K. Effect of NaF addition to mold flux on cuspidine primary field. ISIJ Int. 2006;46:1660–7. https://doi.org/10.2355/isijinternational.46.1660.
Hanao M, Kawamoto M, Watanabe T. Influence of Na2O on phase relation between mold flux composition and cuspidine. ISIJ Int. 2004;44:827–35. https://doi.org/10.2355/isijinternational.44.827.
Pan X, Cui W, Zhang C, Yu H. Formation kinetics and transition mechanism of CaO·SiO2 in low-calcium system during high-temperature sintering. J Cent South Univ. 2020;27:3269–77. https://doi.org/10.1007/s11771-020-4545-1.
Weisweiler W, Osen E, Eck J. Kinetic studies in the CaO-SiO2-System Part I Mechanism and kinetics data of the reactions between CaO- and SiO2- powder compacts. Cement Concrete Res. 1986;16:283–95. https://doi.org/10.1016/0008-8846(86)90103-1.
Saberi A, Golestani FF, Willert PM, Negahdari Z, Liebscher C, Gossler B. A novel approach to synthesis of nanosize Mg2AlO4 spinel powder through sol-gel citrate technique and subsequent heat treatment. Ceram Int. 2009;35:933–7. https://doi.org/10.1016/j.ceramint.2008.03.011.
Stobierski L, Gubernat A. Sintering of silicon carbide I. Effect of carbon Ceram Int. 2003;29:287–92. https://doi.org/10.1016/S0272-8842(02)00117-7.
Wen Y, Liu X, Chen X, Jia Q, Yu R, Ma T. Effect of heat treatment conditions on the growth of MgAl2O4 nanoparticles obtained by sol-gel method. Ceram Int. 2017;43:15246–53. https://doi.org/10.1016/j.ceramint.2017.08.061.
Yang L, Xiao G, Ding D, Li P, Lv L, Yang S. Solid-phase synthesis of MgAl2O4 powder in reducing atmosphere: effects of alumina sources and addition of carbon black. Mater Res Express. 2019;6:045007. https://doi.org/10.1088/2053-1591/aaf967.
Kim J, Lee Y, Lee H. Decomposition of Na2CO3 by interaction with SiO2 in mold flux of steel continuous casting. ISIJ Int. 2001;41:116–23. https://doi.org/10.2355/isijinternational.41.116.
Kawamoto M, Nakajima K, Kanazawa T, Nakai K. Design principles of mold powder for high speed continuous casting. ISIJ Int. 1994;34:593–8. https://doi.org/10.2355/isijinternational.34.593.
Kim J, Lee H. Thermal and carbothermic decomposition of Na2CO3 and Li2CO3. Metall Mater Trans B. 2001;32:17–24. https://doi.org/10.1007/s11663-001-0003-0.
Acknowledgements
The authors wish to thank Chongqing University (in China) for the financial support.
Funding
This study was funded by Chongqing University (in China).
Author information
Authors and Affiliations
Contributions
Fuhang Chen, Guanghua Wen, and Ping Tang contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Fuhang Chen, Liang Yu, and Funian Han. The first draft of the manuscript was written by Fuhang Chen and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
This is an observational study. The Research Ethics Committee has confirmed that no ethical approval is required.
Consent to participate
Not acceptable.
Consent for publication
Not acceptable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, F., Wen, G., Tang, P. et al. The role of carbonaceous materials in mold powder and influence on melting behavior. J Therm Anal Calorim 147, 10965–10975 (2022). https://doi.org/10.1007/s10973-022-11361-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11361-0