Abstract
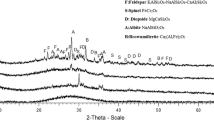
The paper presents and discusses the results of raw materials investigations based on calcium sulphate derived from various origins. The aim of these investigations was the evaluation of perspectives of using flue gas desulfurization gypsum (FGDG) as a potential raw material in the fertilizer industry, in comparison with the currently applied raw materials and others (anhydrite, natural gypsum, phosphogypsum, synthetic building plaster). To preview a new potential industrial application of FGDG, the investigations were focused on thermal analysis (thermogravimetry, differential scanning calorimetry) coupled with mass spectrometry, X-ray fluorescence, Fourier transform infrared spectroscopy and inductively coupled plasma optical emission spectroscopy. The chemical composition and thermal analysis of FGD gypsums in comparison with other samples based on CaSO4 revealed their high purity with low concentration of impurities and heavy metals. Only for one FGDG sample, the concentration of mercury exceeded the acceptable limit. The FGD gypsums seem to be attractive raw materials in the fertilizer industry.










Similar content being viewed by others
References
Lou W, Guan B, Wu Z. Dehydration behavior of FGD gypsum by simultaneous TG and DSC analysis. J Therm Anal Calorim. 2011;104:661–9.
Engbrecht DC, Hirschfeld DA. Thermal analysis of calcium sulfate dehydrate sources used to manufacture gypsum wallboard. Thermochim Acta. 2016;639:173–85.
Gorbovskiy KG, Ryashko AI, Kazakov AI, Norov AM, Mikhaylichenko AI. The influence of water-soluble impurities on thermal dehydration kinetics of phosphogypsum in self-generated atmosphere. J Therm Anal Calorim. 2018;133:1549–62.
De Korte A (2015) Hydration and thermal decomposition of cement/calcium sulphate based materials, Doctoral dissertation.
Galos K, Szlugaj J, Burkowicz A. Sourses of limestone sorbents for flue gas desulphurization in Poland in the context of the needs of domestic power industry. Energy Policy J. 2016;19(2):149–70.
Cordoba P. Status of Flue Gas Desulphurization (FGD) system from coal-fired power plants: overview of the physic-chemical control processes of wet limestone FGDs. Fuel. 2015;144:274–86.
Koralegedara NH, Pinto PX, Dionysiou DD, Al-Abed SR. Resent advances in flue gas desulfurization gypsum processes and applications – A review. J Environ Manage. 2019;251:109572.
Scheinherrova L, Dolezelova M, Havlin J, Trnik A. Thermal analysis of ternary gypsum-based binders stored in different environments. J Therm Anal Calorim. 2018;133:177–88.
Pang M, Sun Z, Huang H. Compressive strength and durability of FGD gypsum-based mortars blended with ground granulated blast furnace slag. Materials. 2020;13:3383.
Phutthimenthakul L, Kumpueng P, Supakata N. Use of flue gas desulfurization gypsum, construction and demolition waste, and oil palm waste trunks to produce concrete bricks. Curr Comput-Aided Drug Des. 2020;10:709.
Wang J, Yang P. Potential flue gas desulfurization gypsum utilization in agriculture: a comprehensive review. Renew Sustain Energy Rev. 2018;82:1969–78.
Presley D (2016) Effects of flue gas desulfurization gypsum on crop yield and soil properties in Kansas, Kansas agricultural experiment station research reports. Vol. 2: Iss. 5.
Chen L, Dick WA (2011) Gypsum as an agricultural amendment: general use guidelines. https://fabe.osu.edu/sites/fabe/files/imce/files/Soybean/Gypsum%20Bulletin.pdf. Accessed 10.09.2021
Uberman R, Naworyta W. The importance of anthropogenic deposits construction for secondary raw materials on the example of synthetic gypsum. Zeszyty Naukowe Instytutu Gospodarki Surowcami Mineralnymi Polskiej Akademii Nauk. 2018;106:211–24.
Szlugaj J, Galos K. Limestone sorbents market for flue gas desulphurization in coal-fired power plants in the context of the transformation of the power industry - A case of Poland. Energies. 2021;14:4275.
Jaworski T, Grochowska S. Circular Economy – the criteria for achieving and the prospect of implementation in Poland. Archiwum Gospodarki Odpadami i Ochrony Środowiska. 2017;19(4):13–22.
Smol M. Transition to circular economy in the fertilizer sector-analysis of recommended directions and end-users’ perception of waste-based products in Poland. Energies. 2021;14:4312.
Chernysh Y, Yakhnenko O, Chubur V, Roubík H. Phosphogypsum recycling: a review of environmental issues, current trends and prospects. Appl Sci. 2021;11:1575.
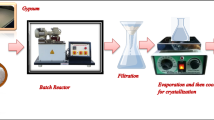
Malinowski P, Biskupski A, Głowiński J. Preparation methods of calcium sulphate and urea adduct. Pol J Chem Technol. 2007;9(4):111–4.
Malinowski P, Borowik M, Wantuch W, Urbańczyk L, Dawidowicz M, Biskupski A. Utilization of waste gypsum in fertilizer production. Pol J Chem Technol. 2014;16(1):45–7.
Sadłowski M, Grzmil B, Lubkowski K, Łuczka K. Separation of urea adducts in the analysis of complex mineral fertilizers. Chem Pap. 2016;70(3):315–24.
Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilizing products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003.
Rutherford PM, Dudas MJ, Arocena JM. Radioactivity and elemental composition of phosphogypsum produced from three phosphate rock sources. Waste Manag Res. 1995;13:407–23.
Caillahua CM, Moura FJ. Technical feasibility for use of FDG gypsum as an additive setting time retarder for Portland cement. J Mater Res Technol. 2018;7(2):190–7.
Zhao S, Duan Y, Lu J, Gupta R, Pudasainee D, Liu S, Liu M, Lu J. Thermal stability, chemical speciation and leaching characteristics of hazardous trace elements in FGD gypsum from coal-fired power plants. Fuel. 2018;231:94–100.
Saadaoui E, Ghazel N, Romdhane BC, Massoudi N. Phosphogypsum: potential uses and problems – a review. Int J Environ Sci. 2017;74(4):558–67.
Liptay G (1975) Atlas of Termochemical curves, Akadémiai Kiadó, Budapest 1975, p. 19.
Biskupski A, Myka A, Borowik M, Malinowski P. Evaluation of reactivity of carbonate filler powders applied in nitrogen fertilizer technology. Przem Chem. 2013;92:1341–5.
Fukami T, Tahara S, Nakasone K, Yasuda Ch. Synthesis, crystal structure and thermal properties of CaSO4·2H2O single crystals. Int J Chem. 2015;7(2):12–20.
Hazzat M, Sifou A, Arsalane S, Hamidi A. Novel approach to thermal degradation kinetics of gypsum: application of peak deconvolution and model-free isoconversional method. J Therm Anal Calorim. 2020;140:657–71.
Strydom CA, Potgieter JH. Dehydration behavior of a natural gypsum and a phosphogypsum during milling. Thermochim Acta. 1999;332:89–96.
Borrachero MV, Paya J, Bonilla M, Monzo J. The use of thermogravimetric analysis technique for the characterization of construction materials. The gypsum case. J Therm Anal Calorim. 2008;91(2):503–9.
Ennaciri Y, Bettach M, Cherrat A, Zegzouti A. Conversion of phosphogypsum to sodium sulfate and calcium carbonate in aqueous solution. J Mater Envir Sci. 2016;7(6):1925–33.
Singh M, Garg M, Verma SK, Kumar R, Kumar H. An improved process for the purification of phosphogypsum. Constr Build Mater. 1996;10(8):597–600.
Barbarossa V, Brutti S, Diamanti M, Sau S, De Maria G. Catalytic thermal decomposition of sulphuric acid in sulphur-iodine cycle for hydrogen production. Int J Hydrog Energy. 2006;31:883–90.
Anderson C, Galwey AK. A kinetic study of the thermal dehydration of calcium sulphite hemihydrate. Can J Chem. 1992;70:2468–75.
Fernandez J, Gonzalez F, Pesquera C, Junior AN, Viana MM, Dweck J. Qualitative and quantitative characterization of a coal power plant waste by TG/DSC/MS, XRF and XRD. J Therm Anal Calorim. 2016;125:703–10.
Zhao L, Wan T, Yang X, Yang L, Kong X, Zhang Z, Wang X. Effects of kaolinite addition on the melting characteristics of the reaction between phosphogypsum and CaS. J Therm Anal Calorim. 2015;119:2119–26.
Lisnic R, Jinga SI. Study on current state and future trends of flue gas desulphurization technologies: a review. Rom J Mater. 2018;48(1):83–90.
Smol M, Kulczycka J. Possibilities in the use of waste as a source of critical raw material in fertilizer sector – implementing the principles of the circular economy (CE) – phosphorus case study (in Polish). W Mikrozanieczyszczenia w ściekach, odpadach i środowisku, Wydawnictwo Politechniki Częstochowskiej. 2018;345(24):331–48.
Hoffmann J, Kaniewski M, Nieweś D, Hoffmann K. Selected magnesium compounds as possible inhibitors of ammonium nitrate decomposition. Pol J Chem Technol. 2020;22(2):1–8.
Sebbahi S, Chameikh ML, Sahban F, Aride J, Benarafa L, Belkbir L. Thermal behavior of Moroccan phosphogypsum. Thermochim Acta. 1997;302:69–75.
Shen Y, Qian J, Chai J, Fan Y. Calcium sulfoaluminate cements made with phosphogypsum: Production issues and material properties. Cem Concr Compos. 2014;48:67–74.
Wagner M (2017) Thermal analysis in practice. Fundamental aspects. p 101.
Ennaciri Y, Bettach M, Cherrat A, Zegzouti A. Conversion of phosphogypsum to sodium sulfate and calcium carbonate in aqueous solution. J Mater Environ Sci. 2016;16:1925–33.
Hass M, Sutherlandt GBBM. The infra-red spectrum and crystal structure of gypsum. Proc R Soc London Ser A Math Phys Sci. 1956;236:427–45.
Prakash KA, Rajdeo SM. Vibrational spectroscopy and SEM-EDX analysis of wall painted surfaces, Orchha Fort. India J Archaeol Sci Rep. 2019;24:434–44.
Holly S, Sohar P. Absorption spectra in the infrared region. Budapest: Akademiai Kiado; 1975.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Myka, A., Łyszczek, R., Zdunek, A. et al. Thermal analysis of materials based on calcium sulphate derived from various sources. J Therm Anal Calorim 147, 9923–9934 (2022). https://doi.org/10.1007/s10973-022-11319-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11319-2