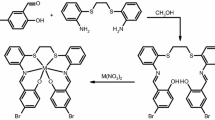
Abstract
Schiff bases are organic compounds that present a broad biological activity, which can be improved by the coordination with different metal ions. Their complexes can also act as corrosion inhibitor agents or even as heterogeneous catalysts in organic reactions. Based on the Schiff base synthesis versatility, and its several potential applications, this work focused on the obtention of a Schiff base derived from 4-aminosalicylic acid and salicylaldehyde by the mechanochemical method, and its Co(II), Cu(II), and Zn(II) complexes by precipitation in an aqueous solution. Using eco-friendly methods, it was not necessary to employ organic solvents or heating in the syntheses. Spectroscopic techniques were used to characterize the structure of the Schiff base and its coordination compounds, among them, MS, FTIR, UV/VIS/NIR, thermoanalytical techniques (TG-DSC, DSC, and EGA), and X-ray powder diffraction. The spectroscopic and thermal results confirmed Schiff base formation, and by the thermoanalytical study, it was possible to evaluate its thermal behavior and propose a thermal degradation mechanism, which results in the formation of a decarboxylation, resulting in the 3-aminophenol, a common thermal product of 4-aminosalicylic acid. For the metal complexes, the analytical and thermoanalytical results showed that they present a 1:1 metal–ligand stoichiometry, while FTIR and UV–VIS/NIR spectra demonstrated that the ligand coordinated with the metals in different ways through the carboxylate group and the phenolic oxygen. Furthermore, the EGA results showed that during the metal complexes decomposition, salicylaldehyde releasing is common for Co(II) and Zn(II) complexes, which is resulted from the C–N bond break.
Graphical abstract









Similar content being viewed by others
References
Kajal A, Bala S, Kamboj S, Sharma N, Saini V. Schiff bases: a versatile pharmacophore. J Catal. 2013;2013:1–15.
Cleiton M, Daniel L, Modolo LV, Alves RB, De Resende MA, Martins CVB. A short review of their antimicrobial activities. J Adv Res. 2011;2:1–8.
Jia Y, Li J. Molecular assembly of Schiff base interactions: construction and application. Chem Rev. 2015;3:1597–621.
Divya K, Pinto GM, Pinto AF. Application of metal complexes of Schiff bases as an antimicrobial drug: a review of recent works. Int J Curr Pharm Res. 2017;9:27–30.
Abu-dief AM, Mohamed IMA. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ J Basic Appl Sci. 2015;4:119–33.
Ikram M, Rehman S, Baker RJ, Ur H, Khan A, Iqbal M. Synthesis and distinct urease enzyme inhibitory activities of metal complexes of Schiff-base ligands: kinetic and thermodynamic parameters evaluation from TG-DTA analysis. Thermochim Acta. 2013;555:72–80.
Ghosh P, Dey SK, Ara MH, Karim K, Nazmul Islam ABM. A review on synthesis and versatile applications of some selected Schiff bases with their transition metal complexes. Egypt J Chem. 2019;62:523–47.
Xie J, Li C, Dong J, Qu W, Pan L, Peng M. The standard molar enthalpy of formation of a new copper (II) Schiff-base complex and its interaction with bovine serum albumin. Thermochim Acta. 2014;598:7–15.
Boman G. Serum concentration and half-life of rifampicin after simultaneous oral administration of aminosalicylic acid or isoniazid. Eur J Clin Pharmacol. 1974;3:217–25.
Bhattacharya B, Haldar R, Dey R, Maji TK, Ghoshal D. Porous coordination polymers based on functionalized Schiff base linkers: enhanced CO2 uptake by pore surface modification. Dalton Trans. 2014;5:2272–82.
Mirza ZA, Saleem M, Sadeeq M, Kosser F, Mirza SI. Preparation and characterization of metal complexes of Tungsten (VI) with biologically active Schiff bases. J Chem Pharm. 2014;6:706–10.
Patole J, Shingnapurkar D, Ratledge C. Schiff base conjugates of p-aminosalicylic acid as antimycobacterial agents. Med Chem Lett. 2006;16:1514–7.
Belz T, Ihmaid S, Al-rawi J, Petrovski S. Antifungal activity of N-(benzyl carbamoyl or carbamothioyl)-2-hydroxy substituted benzamide and 2-benzyl amino-substituted benzoxazines. Int J Med Chem. 2013;1:1–20.
Ashraf MA, Wajid A, Mahmood K, Maah MJ, Yusoff I. Spectral investigation of the activities of amino substituted bases. Orient J Chem. 2011;27:27–31.
Anderson ABC, De Carvalho GSG, Marques LF, Correa CC, Da Silva AD, et al. The zwitterionic structure of 2-hydroxy-4-[(2-hydroxybenzylidene)amino]benzoic acid. Acta Crystallogr. 2013;69:934–6.
El-Haty MT, Adam FA, Nadia AA. Coordination of Y(lU), Ce(tII), La(IU). AN)) Zr(1V) with some salicylidene aromatic Schiff bases. J Chin Chem Soc. 1984;31:49–54.
James SL, Adams CJ, Bolm C, Braga D, Collier P, Jones W. Mechanochemistry: opportunities for new and cleaner synthesis. Chem Soc Rev. 2012;41:413–47.
De Moura A, Gaglieri C, Da Silva-Filho LC, Caires FJ. Mechanochemical synthesis, characterization and thermoanalytical study of a new curcumin derivative. J Therm Anal Calorim. 2021;146:587–94.
De Moura A, Gaglieri C, Alarcon TR, Ferreira TL, Vecchi R, et al. A new curcuminoids-coumarin derivative: mechanochemical synthesis, characterization and evaluation of its in vitro cytotoxicity and antimicrobial properties. ChemistrySelect. 2021;6:11352–61.
Do J-L, Friščić T. Mechanochemistry: a force of synthesis. ACS Cent Sci. 2017;3:13–7.
De Oliveira CN, Ionashiro M, Graner CAF. Titulação complexométrica de zinco cobre e cobalto. Eclética Quím. 1985;10:7–10.
Silverstein RM, Webster FX. Spectrometric identification of organic compounds. 7th ed. Hoboken: Wiley; 2005.
Wani AB, Singh J, Upadhyay N. Synthesis and characterization of transition metal complexes of para-aminosalicylic acid with evaluation of their antioxidant activities. Orient J Chem. 2017;30:1120–6.
Rotich MK, Glass BD, Brown ME. Thermal studies on some substituted aminobenzoic acids. J Therm Anal. 2001;64:681–8.
Parmar B, Kumar K, Maiti P, Paul P. Syntheses, characterization and crystal structures of potassium and barium complexes of a Schiff base ligand with different anions. J Chem Sci. 2014;126:1373–84.
Dippong T, Stoia M. Study on the obtaining of cobalt oxides by thermal decomposition of some complex combinations, undispersed and dispersed in SiO2 matrix. J Therm Anal Calorim. 2008;94:389–93.
Iwase K, Nagano Y, Yoshikawa I, Houjou H, Yamamura Y, Saito K. Cold crystallization in Schiff-base nickel (II) complexes derived from three toluidine isomers. J Phys Chem. 2014;118:27664–71.
De Roo J, Baquero EA, Coppel Y, De Keukeleere K. Insights into the ligand shell, coordination mode, and reactivity of carboxylic acid capped metal oxide nanocrystals. Chem Plus Chem. 2016;81:1–9.
Cortijo M, Herrero S, Jimenez-Aparicio R, Perles J, Priego JL, Torralvo MJ, Torroba J. Hybrid polyfunctional systems based on nickel (II) isonicotinate. Eur J Inorg Chem. 2013;14:2580–90.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds, part B: applications in coordination, organometallic, and bioinorganic chemistry. 6th ed. Hoboken: Wiley; 2009.
Panicker CY, Varghese HT, John A, Philip D, Istvan K, Keresztury G. FT-IR, FT-Raman and FT-SERS spectra of 4-aminosalicylic acid sodium salt dihydrate. Spectrochim Acta A Mol Biomol Spectrosc. 2002;58:281–7.
Wang YA, Liu T, Zhong GQ. Synthesis, characterization and applications of copper(II) complexes with Schiff bases derived from chitooligosaccharide and iodosubstituted salicylaldehyde. Carbohydr Polym. 2019;224:115151–1151158.
Tian Y, Allan P, He X, McPherson LJM. Synthesis and structural characterization of a single-crystal to single-crystal transformable coordination polymer. Dalton Trans. 2013;4:3–7.
Pretsch T, Chapman KW, Halder GJ, Kepert CJ. Dehydration of the nanoporous coordination framework Er III [Co III (CN) 6] 4 (H2O): single crystal to single crystal transformation and negative thermal expansion in Er III [Co III (CN) 6]. Chem Comm. 2006;4:1857–9.
Lampman GM, Kriz GS, Vyvyan JR. Introduction to spectroscopy. 5ª. New York: Cengage Learning; 2014.
De Godoi R, Gaglieri C, Turra RA, De Moura A, De Almeida AC, Caires FJ, Ionashiro M. Cobalt selenate pentahydrate: thermal decomposition intermediates and their properties dependence on temperature changes. Thermochim Acta. 2020;689:178615–22.
Ahmad F, Aly EH, Atef M, Elokr MM. Study the influence of zinc oxide addition on cobalt doped alkaline earth borate glasses. Alloys Compd. 2014;593:250–5.
Farouk M, Ahmad F, Samir A. Ligand field and spectroscopic investigations of cobalt doped erbium–zinc borate glasses. Opt Quantum Electron. 2019;292:1–12.
Lever ABP. Inorganic electronic spectroscopy. New York: Elsevier; 1984.
Kawata S, Heise HM. Near-infrared spectroscopy: principles, instruments, applications. Hoboken: Wiley VHC; 2002.
Workman J, Weyer L. Practical guide to interpretive near-infrared. Boca Raton: CRC Press; 2007.
Pouramini Z, Moradi A. Structural and orthoselectivity study of 2-hydroxybenzaldehyde using spectroscopic analysis. Arab J Chem. 2012;5:99–102.
Acknowledgements
The authors would like to thank FAPESP (Grant Numbers 2018/24378-6 and 2021/05926-5), CNPq (Grant Numbers 317282/2021-2 and 422893/2021-8) and to CEPID-CDMF laboratory (Grant Number 2013/07296-2) for the X ray powder diffraction.
Author information
Authors and Affiliations
Contributions
AM contributed to methodology, conceptualization, validation, formal analysis, investigation, writing—original draft; writing—review and editing; JBJ contributed to methodology, formal analysis, writing—original draft; ACSC contributed to conceptualization, formal analysis, and investigation; FJC contributed to conceptualization, funding acquisition, project administration, resources, supervision, writing—review and editing.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 2 (MP4 4959 kb)
Supplementary file 3 (MP4 11828 kb)
Supplementary file 4 (MP4 16420 kb)
Supplementary file 5 (MP4 16137 kb)
Supplementary file 6 (MP4 18835 kb)
Rights and permissions
About this article
Cite this article
de Moura, A., Júnior, J.B., Carvalho, A.C.S. et al. Green synthesis of a Schiff base ligand and its Co(II), Cu(II) and Zn(II) complexes: thermoanalytical and spectroscopic studies. J Therm Anal Calorim 147, 11093–11106 (2022). https://doi.org/10.1007/s10973-022-11293-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11293-9