Abstract
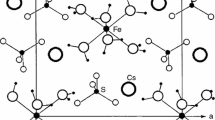
The thermal behavior of salts of the type [Co(NH3)6]X3 [X = Cl, Br, I] has been the object of considerable interest since early in the twentieth century. As noted, some of the early measurements on the trichloride complex recorded what appeared to be an “anomaly” occurring in its heat capacity. Our studies, however, indicate a far more complex behavior in which (a) DSC measurements between 100 and 300 K show a glass transition at ca. 200 K corresponding to the previously-mentioned “anomaly”; (b) the glass transition is observed in both the heating and cooling cycles, but the exact details are dependent on the rate at which the process is carried out in either direction; (c) parallel crystallographic studies using samples from the same batches as those in the DSC studies document that the origin of the thermal changes are due to torsional motions of the –NH3 hydrogens about the Co–N vector, evidence of the formation of a complex structure (condis crystalline mesophase) with a variety of order–disorder (entropically-diverse) material of differing stability and mobility; (d) inasmuch as there are four independent [Co(NH3)6]+ cations in the asymmetric unit (Z’ = 3), an additional reason for the complexity of the DSC observations is due to the sequential degree of torsional disorder induced thermally during the heating cycle on each of the four individual specimens of the asymmetric unit; (e) similar behavior is observed in the cooling cycle, but, the “re-orientation” of the thermally excited –NH3 ligands to a “cooler” more-orderly condition is considerably more sluggish (hysteresis)—thus, the observed differences in the recorded rates mentioned above; (f) finally, and not surprisingly, observations carried out with different crystals of (1) the same batch and (2) different batches show the same general behavior, but the specific details vary considerably, indicating that the history (provenance) of the crystal preparation is crucial to the exact results observed in each case.






Similar content being viewed by others
References
Werner A. Beitrag zur konstitution anorganischer verbindungen. Z Anorg Chem. 1893;3:267–330.
Werner A. Zur Kenntnis des asymmetrischen Kobaltatoms. Ber Dtsch Chem Ges. 1911;44:1887–98.
Werner A, Miolati A. Beitrage zur Konstitution anorganischer Verbindungen. Z Phys Chem. 1893;12:35–55.
Werner A, Miolati A. Gazz Chim Ital. 1893;23:140–65.
Werner A, Miolati A. Beiträge zur Konstitution anorganischer Verbindungen II Abhandlung. Z Phys Chem. 1894;14:506–21.
Werner A, Miolati A. Gazz Chim Ital. 1894;24:408–27.
Werner A, Miolati A. Beiträge zur Konstitution anorganischer Verbindungen III. Z Phys Chem. 1896;21:225–38.
Werner A, Miolati A. Gazz Chim Ital. 1896;27:299–316.
Ziegler WT. The heat capacities of some cobalt hexammine halides. J Amer Chem Soc. 1941;63:2700–3.
Timmermans J. Plastic crystals: a historical review. J Phys Chem Solids. 1961;18:1–8.
Bjerrum J, McReynolds JP. Hexamminecobalt(III) Salts. Inorg Synth. 1946;2:216–21. https://doi.org/10.1002/9780470132333.ch69.
Fremy ME. Recherches sur le cobalt. Annales de chimie et de physique. 1852;35:257–312.
Kluiber RW, Rutgers University, Newark, NJ. (2014). Private Communication
Clayton PR, Staveley AK, Weir RD. A calorimeter for heat capacity measurements of high precision from 1.4 to 100 K. The heat capacity of hexa-ammine cobalt (III) chloride from 2.1 to 309K. J Chem Phys. 1981;76:5464–73.
Wunderlich B. Thermal Analysis, Academic Press Inc., 1250 Sixth Avenue, San Diego, CA 92101, (1990), ISBN 0–12–755605–7.
Wunderlich B. The detection of conformational disorder by thermal analysis. Pure Appl Chem. 1989;61(8):1347–51.
Wunderlich B, Grebowicz J. Thermotropic mesophases and mesophase transitions of linear flexible macromolecules. Adv Polym Sci. 1984;60–1:1–59.
Wunderlich B, Grebowicz J. Do condis crystals exist? Polym Preprints (ACS). 1983;24:290–1.
Grebowicz J, Wunderlich B. On Cp to Cv conversion for solid linear macromolecules. J Thermal Anal. 1985;30:229–36.
Wunderlich B, Möller M, Wiedemann HG. Condis crystals of small molecules I. The concept and limitations. Mol Cryst Liquid Cryst. 1986;140(2–4):211–8. https://doi.org/10.1080/00268948608080154.
Wunderlich B. The Nature of the Glass Transition and its Determination by Thermal Analysis. In: Seyler RJ, editor. Assignment of the Glass Transition. 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959: ASTM International; 1994. p. 17-17–15. https://doi.org/10.1520/STP15363S.
Bruker SADABS, SAINT and SMART. Bruker AXS, Inc., Madison, Wisconsin, USA; 2008
Sheldrick GM. SHELXT – Integrated space-group and crystal-structure determination. Acta Cryst Sect A Found Adv. 2015;71(1):3–8. https://doi.org/10.1107/S2053273314026370.
Sheldrick G M. Crystal structure refinement with SHELXL. Acta Cryst Sect C Struct Chem. 2015;71(1):3–8. https://doi.org/10.1107/S2053229614024218.
Putz H, Brandenburg K. DIAMOND Version 8.5.10. GbR, Kreuzherrenstr. 102, 5322 Bonn; 2019
CSD = Cambridge Structural Database CCSD, Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ UK, Tel = +44–1223–336408. They can be contacted at http://www.ccdc.cam.ac.uk; 2019
Acknowledgements
The authors acknowledge the National Science Foundation for Science and Technology Development (grant No. 0443538), for part of the purchase of the X-ray diffractometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bernal, I., Lalancette, R.A., Syzdek, D. et al. The effect of temperature on the phase structure and transitions of [Co(NH3)6]Cl3 from 100 K until its decomposition. J Therm Anal Calorim 147, 11119–11125 (2022). https://doi.org/10.1007/s10973-022-11291-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11291-x