Abstract
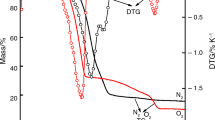
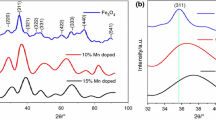
NixCo1−xFe2O4 (x = 0, 0.5, and 1) spinel nanoparticles (NPs) have been prepared using the ceramic method and Ni0.5Co0.5Fe2O4 using green, microwave, and sol–gel routes. The as-prepared materials were characterized by XRD, FT-IR, SEM TEM, and N2 adsorption/desorption techniques. The catalytic activity, thermal stability, and kinetic parameters of the effect of synthesized materials upon thermal decomposition of ammonium perchlorate (AP) were studied through differential scanning calorimetry (DSC) and thermogravimetric techniques (TG). The addition of 2 mass% of nanosized ferrites to AP shifted the thermal degradation temperature of AP to lower temperatures. The catalytic activity for AP thermal degradation followed the order: NiCoF-gl > NiCoF-gr > NiCoF-mic > NiCoF-cer > NiF > CoF. The kinetic parameters for the ferrite-catalyzed reaction, using the isoconversional methods for NiCoF-gl, showed a decrease in the activation energy and preexponential factor of ammonium perchlorate thermal dissociation compared with the uncatalyzed one. The thermokinetic parameters for the catalytic decomposition process were determined and a mechanism was suggested for the kinetic reaction. The DSC results showed that the decomposition temperatures of AP decreased with the addition of the ferrites. The heat releases of the AP/ferrite mixtures were 0.82, 0.89, 0.91, 0.98, 1.04 and 1.23 kJ g−1 of AP, for CoF, NiF, NiCoF-cer, NiCoF-gr, NiCoF-mic, and NiCoF-gl, respectively, compared to 0.72 kJ g−1 for neat AP.










Similar content being viewed by others
References
Campos EA, Pinto DVBS, Oliveira JLS de, Mattos EdaC, Dutra RdeCL. Synthesis, characterization and applications of iron oxide nanoparticles. J Aerosp Technol Manage. 2015;7: 267–76.
Yadav N, Srivastava PK, Varma M. Recent advances in catalytic combustion of AP-based composite solid propellants. J Defence Technol. 2020. https://doi.org/10.1016/j.dt.2020.06.007.
Rao DCK, Yadav N, Joshi PC. Cu–Co–O nano-catalysts as a burn rate modifier for composite solid propellant. J Defence Technol. 2016;12:297–304.
Brewster MQ, Mullen JC. Burning-rate behavior in aluminized wide-distribution AP composite propellants. Combust Explos Shock Waves. 2011;47:200–8.
Srivastava P, Dubey R, Kapoor PS, Singh G. Synthesis, characterization and catalytic effect of nimetallic nanocrystals on the thermal decomposition of ammonium perchlorate. Indian J Chem. 2010;49A:1339–44.
Sinditskii VP, Egorshev VY. Combustion mechanism and kinetics of thermal decomposition of ammonium chlorate and nitrite: combustion mechanism and kinetics of thermal decomposition. Centr Eur J Energ Mater. 2010;7:61–75.
Balzer JE, Siviour CR, Walley SM, Proud WG, Field JE. Behaviour of ammonium perchlorate-based propellants and a polymer-bonded explosive under impact loading. J Proc R Soc A Math Phys Eng Sci. 2004;460:781–806.
Chaturvedi S, Dave PN. Solid propellants: AP/HTPB composite propellants. Arab J Chem. 2019;12:2061–8.
Xiao X, Peng B, Cai L, Zhang X, Liu S, Wang Y. The high efficient catalytic properties for thermal decomposition of ammonium perchlorate using mesoporous ZnCo2O4 rods synthesized by oxalate co-precipitation method. J Sci Rep. 2018. https://doi.org/10.1038/s41598-018-26022-2.
Han A, Liao J, Ye M, Li Y, Peng X. Preparation of nano-MnFe2O4 and its catalytic performance of thermal decomposition of Ammonium perchlorate. Chin J Chem Eng. 2011;19(6):1047–51.
Li G, Bai W. Synthesis of hierarchical flower-likeCo3O4superstructure and its excellent catalytic property for ammonium perchlorate decomposition. J Chem Phys. 2018;506:45–51.
Chen L, Li L, Li G. Synthesis of CuO nanorods and their catalytic activity in the thermal decomposition of ammonium perchlorate. J Alloy Compd. 2008;464(1):532–6.
Wang Y, Zhu J, Yang X, Lu L, Wang X. Preparation of NiO nanoparticles and their catalytic activity in the thermal decomposition of ammonium perchlorate. Thermochim Acta. 2005;437(1):106–9.
Zhang Y, Ma M, Zhang X, Wang B, Liu R. Synthesis, characterization, and catalytic property of nanosized MgO flakes with different shapes. J Alloys Compd. 2014;590:373–9.
Zhang Y, Liu X, Nie J, Yu L, Zhong Y, Huang C. Improve the catalytic activity of a-Fe2O3 particles in decomposition of ammonium perchlorate by coating amorphous carbon on their surface. J Solid State Chem. 2011;184(2):387–90.
Juibari NM, Eslami A. Investigation of catalytic activity of ZnAl2O4 and ZnMn2O4 nanoparticles in the thermal decomposition of ammonium perchlorate. J Therm Anal Calorim. 2017;128(1):115–24.
Aijun H, Juanjuan L, Mingquan Y, Yan L, Xinhua P. Preparation of nano-MnFe2O4 and its catalytic performance of thermal decomposition of ammonium perchlorate. Chin J Chem Eng. 2011;19(6):1047–51.
Hosseini SG, Abazari R, Gavi A. Pure CuCr2O4 nanoparticles: synthesis, characterization and their morphological and size effects on the catalytic thermal decomposition of ammonium perchlorate. J Solid State Sci. 2014;37:72–9.
Jacobs PWM, Whitehead HM. Decomposition and Combustion of ammonium perchlorate. J Chem Rev. 1969;69:551–90.
Cui B, Lin H, Li JB, Li X, Yang J, Tao J. Core-ring structured NiCo2O4 nanoplatelets: synthesis, characterization, and electrocatalytic applications. J Adv Funct Mater. 2008;18:1440–7.
Singh G, Kapoor IPS, Dubey R, Srivastava P. Preparation, characterization and catalytic behavior of CdFe2O4 and Cd nanocrystals on AP. HTPB and composite solid propellants, Part:79. Thermo-chim Acta. 2015;1–2(511):112–8.
Wei SH, Zhang SB. First-principles study of cation distribution in eighteen closed-shell AIIB2IIIO4 and AIVB2IIO4 spinel oxides. J Phys Rev B. 2001;63(4):045112.
Jacobs JP, Maltha A, Reintjes JGH, Drimal J, Ponec V, Brongersma HH. The surface of catalytically active spinels. J Catal. 1994;147(1):294–300.
Vozniuk O, Tabanelli T, Tanchoux N, Millet Jean-Marc M, Albonetti S, Di Renzo F, Cavani F. Mixed-oxide catalysts with spinel structure for the valorization of biomass: the chemical-loop reforming of bioethanol. Catalysts. 2018;8:332. https://doi.org/10.3390/catal8080332.
Xiao X, Peng B, Cai L, Zhang X, Liu S, Wang Y. The high efficient catalytic properties for thermal decomposition of ammonium perchlorate using mesoporous ZnCo2O4 rods synthesized by oxalate co-precipitation method. Sci Rep. 2018;8:7571.
Modanlou N, Tarighi S. MnC2O4 nanoparticles with excellent catalytic activity in thermal decomposition of ammonium perchlorate. J Therm Anal Calorim. 2018;133:1317–26.
Chen T, Ping D, Jiang W, Liu J, Hao G, Gao H, Xiao L, Ke X, Zhao F, Xuan C. A facile one-pot solvothermal synthesis of CoFe2O4/RGO and its excellent catalytic activity on thermal decomposition of ammonium perchlorate. R Soc Chem Adv. 2016;6:83838–47.
Alizadeh-Gheshlaghi E, Shaabani B, Khodayari A, Azizian-Kalandaragh Y, Rahimi R. Investigation of the catalytic activity of nano-sized CuO, Co3O4 and CuCo2O4 powders on thermal decomposition of ammonium perchlorate. Powder Technol. 2012;217:330–9.
Gheshlaghi EA, Shaabani B, Khodayari A, Kalandaragh YA, Rahimi R. Investigation of the catalytic activity of nano-sized CuO, Co3O4and CuCo2O4 powders on thermal decomposition of ammonium perchlorate. Powder Technol. 2012;217:330–9.
Singh G, Kapoor IPS, Dubey S, Siril PF. Preparation characterization and catalytic activity of transition metal oxide nanocrystals. J Sci Con Proc. 2008;1(7):11–7.
Zhang X, Zheng J, Fangb H, Zhang Y, Bai S, He G, Li K. Catalytic decomposition and crack resistance of composite energetic material synthesized by recrystallizing with graphene oxide. Compos A Appl Sci Manuf. 2009;118:90–8.
Chaturvedi S, Dave PN, Patel NN. Thermal decomposition of AP/HTPB propellants in presence of Zn nanoalloys. J Appl Nano Sci. 2015;5:93–8.
Joshi SS, Paul PR, Krishnamurthy VN. Thermal decomposition of ammonium perchlorate in the presence of nano sized ferric oxide. J Defence Sci. 2008;58(6):721–7.
Lucas E, Decker S, Khaleel A, Seitz A, Futlz S, Ponce A, Li W, Carnes C, Klabunde KJ. Nanocrystalline metal oxides as unique chemical reagents/sorbents. Chem A Eur J. 2001;7(12):2505–10.
Venkatachalam V, Alsalme A, Alghamdi A, Jayavel R. Hexagonal-like NiCo2O4 nanostructure based high-performance supercapacitor electrodes. J Ionics. 2017;23:977–84.
Velmurugan M, Chen SM. Synthesis and characterization of porous MnCo2O4 for electrochemical determination of cadmium ions in water samples. J Sci Rep. 2017;7:66. https://doi.org/10.1038/s41598-017-00748-x.
Košak A, Makovec D, Drofenik M. The preparation of MnZn-ferrite nanoparticles in a water/CTAB, 1-butanol/1-hexanol reverse microemulsion. J Phys Status Solidi C. 2004;1(12):3521–4.
Khairy M, El-Shaarawy MG, Mousa MA. Characterization and super-capacitive properties of nanocrystalline copper ferrite prepared via green and chemical methods. J Mater Sci Eng . 2021;263:114812.
Naghikhani R, Nabiyouni G, Ghanbari D. Simple and green synthesis of CuFe2O4–CuO nanocomposite using some natural extracts: photo-degradation and magnetic study of nanoparticles. J Mater Sci Mater Electron. 2018;29:4689–703.
Jalajerdi R, Ghanbari D. Microwave synthesis and magnetic investigation of CuFe2O4 nanoparticles and poly styrene-carbon nanotubes composites. J Nanostruct. 2016;26:278–84.
Costa AF, Pimentel PM, Aquino FM, Melo DMA, Melo MAF, Santos IMG. Gelatin synthesis of CuFe2O4 and CuFeCrO4 ceramic pigments. J Mater Lett. 2013;112:58–61.
Khawam A, Flanagan DR. Solid-state kinetic models: Basics and mathematical fundamentals. J Phys Chem B. 2006;110:17315–28.
Casal MD, Marbán G. Combined kinetic analysis of solid-state reactions: the integral method (ICKA). Int J Chem Kin. 2020. https://doi.org/10.1002/kin.21416.
Ninan KN, Krishnan K, Krishnamurthy VN, Ir ley J. Kinetics and mechanism of thermal decomposition of in situ generated calcium carbonate. J Therm Anal. 1991;37:1533–43.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Appl Pyrolys. 2008;81:253–62.
Atanassov A, Genieva S, Vlaev L. Study on the thermo oxidative degradation kinetics of tetrafluoroethylene-ethylene copolymer filled with rice husks ash. J Polym Plast Technol Eng. 2010;49:541–54.
Khawam A, Flanagan DR. Basics and applications of solid-state kinetics: a pharmaceutical perspective. J Pharm Sci. 2006;95:472–98.
Ravi P, Vargeese AA, Tewari SP. Isoconversional kinetic analysis of decomposition of nitropyrazoles. Thermochim Acta. 2012;550:83–9.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun. 2006;27:1515–32.
Kissinger HE. Reaction kinetics in differential thermal analysis. J Anal Chem. 1957;29:1702–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Them Analy. 1970;2:301–24.
Janković B, Smičiklas I. The non-isothermal combustion process of hydrogen peroxide treated animal bones. Kinetic analysis. Thermochim Acta. 2011;521:130–8.
Dhyani V, Kumar J, Bhaskar T. Thermal decomposition kinetics of sorghum straw via thermogravimetric analysis. J Bioresource Technol. 2017;45:1122–9.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry application to a phenolic plastic. J Polym Sci C Polym Symp. 1964;6:66. https://doi.org/10.1002/polc.5070060121.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Therm Anal. 1977;11:445–7.
Valanciene E, Miknius L, Pedisius N. The influence of zeolite catalyst on kinetics and thermodynamics of polypropylene waste thermal degradation. J Therm Anal Calorim. 2016;124:341–54.
Genieva S, Vlaev L, Atanassov A. Study of the thermooxidative degradation kinetics of poly (tetrafluoroethene) using iso-conversional calculation procedure. J Therm Anal Calorim. 2010;99(2):551–61.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1–2):1–19.
Ghuge NS, Mandal D. Synthesis of LiDyO2 by solid-state reaction process and study of reaction kinetics by using TG-DTA and XRD techniques. Indian Chem Eng. 2017;59(2):101–16.
Khairy M, Bayoumy WA, Selima SS, Mousa MA. Studies on characterization, magnetic and electrochemical properties of nano-size pure and mixed ternary transition metal ferrites prepared by the autocombustion method. J Mater Res. 2020. https://doi.org/10.1557/jmr.2020.200.
Islam N, Ghosh TB, Chopra KL, Acharya HN. XPS and X-ray diffraction studies of aluminum-doped zinc oxide transparent conducting films. J Thin Solid Films. 1996;280:20–5.
Srinivasan TT, Srivastava CM, Venkataramani N, Patni MJ. Infrared absorption in spinel ferrites. Bull Mater Sci. 1984;6:1063–7.
Patil RP, Deleka SD, Mane DR, Hankare PP. Synthesis, structural and magnetic properties of different metal ion substituted nanocrystalline zinc ferrite. J Results Phys. 2013;3:129–33.
Şabikoğlu I, Paral L, Malina O, Novak P, Kaslik J, Tucek J, Pechousek J, Navarik J, Schneeweiss O. The effect of neodymium substitution on the structural and magnetic properties of nickel ferrite. Prog Nat Sci Mater Int. 2015;25:215–21.
Kumar H, Singh JP, Srivastava RC, Negi P, Agrawal HM, Asokan K. FTIR and electrical study of dysprosium doped cobalt ferrite nanoparticles. J Nano Sci. 2014. https://doi.org/10.1155/2014/862415.
Pradeep A, Priyadharsini P, Chandrasekaran G. Sol–gel route of synthesis of nanoparticles of MgFe2O4 and XRD, FTIR and VSM study. J Magn Magn Mater. 2008;320(21):2774–9.
Compos EA, Fernandes MTC, Kawachi EY, Oliveira JIS, Dutra RCL. Chemical and textural characterization of iron oxide nanoparticles and their effect on the thermal decomposition of ammonium perchlorate. J Propell Explor Pyrotechnol. 2015;40(6):860–6.
Juibari MN, Tarighi S. MnCo2O4 nanoparticles with excellent catalytic activity in thermal decomposition of ammonium perchlorate: green synthesis and kinetic study. J Therm Anal Calorim. 2018;133:1317–26.
Shim HM, Lee EA, Kim JK, Kim HS, Koo KK. Formation of tungsten/ammonium perchlorate composites and their reaction kinetics. Cent Eur J Energ Mater. 2015;12:703–22.
Ding Y, Ezekoye OA, Lu S, Wang C, Zhou R. Comparative pyrolysis behaviors and reaction mechanisms of hardwood and softwood. J Energy Convers Manag. 2017;132(15):102–9.
Joshi SS, Paul PR, Krishnamurthy VN. Thermal decomposition of ammonium perchlorate in the presence of nanosized ferric oxide. J Defence Sci. 2008;58:721–7. https://doi.org/10.14429/dsj.58.1699.
Jacobs PMW, Russell-Jones A. On the mechanism of the decomposition of ammonium perchlorate. J Aerosp Res Cent. 2012. https://doi.org/10.2514/3.4085.
Essel JT, Nelson AP, Smilowitz LB, Henson BF, Merriman LR, Turnbaugh D, Gray C, Shermer KB. Investigating the effect of chemical ingredient modifications on the slow cook-off violence of ammonium perchlorate solid propellants on the laboratory scale. J Energ Mater. 2020;38:127–41.
Wang Y, Song X, Li F. Thermal behavior and decomposition mechanism of ammonium perchlorate and ammonium nitrate in the presence of nanometer triaminoguanidine nitrate. J ACS Omega. 2019;4:214–25.
Xiao X, Zhang Z, Cai L, Li Y, Yan Z, Wang Y. The excellent catalytic activity for thermal decomposition of ammonium perchlorate using porous CuCo2O4 synthesized by template-free solution combustion method. J Alloys Compd. 2019;797:548–57.
Turmanova S, Genieva S, Vlaev L. Kinetics of nonisothermal degradation of some polymer composites: change of entropy at the formation of the activated complex from the reagents. J Thermodyn. 2011;66:1–10. https://doi.org/10.1155/2011/605712.
Vara A, Dave Chaturvedi S. The catalytic activity of transition metal oxide nanoparticles on thermal decomposition of ammonium perchlorate. J Defence Technol. 2019;15:629–35.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kammar, E.A., Gad, E.A.M. & Mousa, M.A. Kinetics of thermal decomposition of ammonium perchlorate with nanocrystals of NixCo1−x Fe2O4 (x = 0, 0.05, and 1) ferrites. J Therm Anal Calorim 147, 8119–8135 (2022). https://doi.org/10.1007/s10973-021-11112-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11112-7