Abstract
We dried the orange slices massed 100 ± 0.10 g from the initial moisture content of 6.97 ± 0.02 kg water kg −1DM to the final moisture ones of 0.12 ± 0.01 kg water kg −1DM using two different drying methods defined as convective drying at 50, 75, 100, and 125 °C along with microwave drying at eight output power between 90 and 1000 W. In the study, we measured the drying methods' energy consumption and observed that the microwave drying method's energy consumption was very low at high and low powers. Also, we modeled the results using twenty-one different thin-layer drying equations and obtained results closest to experimental data with the modified Henderson and Pabis equation for all powers in microwave drying and all temperatures in convective drying. We calculated both effective moisture diffusivities and activation energy using the drying data. Some thermal properties such as specific heat, thermal conductivity, thermal diffusivity, and thermal effusivity were calculated and recorded to be decreasing in all thermal properties with drying. Also, we measured the color parameters known as L, a, b, C, α°, and ΔE, browning index (BI), whitening index (WI), and vitamin C (ascorbic acid) in the study. We concluded that the most suitable drying method is microwave drying at medium powers of 350 and 500 W by considering both drying and quality parameters.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orange (Citrus sinensis L.) belongs to the family Rutaceae and is usually preferred as fresh [1] and juice due to its sour–sweet and refreshing taste [2]. It is one of the most grown, consumed, and traded fruits globally [3]. Also, orange has antioxidant, anticancer, and anti-inflammatory effects thanks to its high concentration of vitamin C, which helps maintain cardiovascular health [4,5,6]. On the other hand, vitamin C, which is abundant in oranges, strengthens the immune system and helps the human metabolism to be resistant to viral diseases such as COVID-19, which are fought around the world since December 2019 [7, 8]. It is also one of the most important sources of dietary fiber [9] and contains phenolic compounds, minerals, and carotenoids [10]. The orange, which is harvested only at the end of autumn and during the winter season, can be preserved by canned, jam, frozen, and dried in order to extend its consumption life [11]. The drying of unusual fruits such as orange is a preservation method that has become increasingly popular recently worldwide.
Drying, which is also called dehydration, can generally be defined as the evaporation of water in the product by both heat and mass transfer [12]. It provides a significant advantage in terms of transportation and storage, as well as allowing the products to be preserved for a long time by reducing the microbiological activity that occurs in agricultural products after harvest [13, 14]. Drying can be carried out using many methods. In convective drying, which is one of the most common methods, although the initial investment and operating costs are low and the system is simple, this method has significant disadvantages such as long drying time and low energy efficiency as well as causing adverse effects on product quality [15,16,17,18]. Due to the disadvantages of convective drying, the use of the microwave drying method has become widespread both in the scientific platform and in the industry in recent years [19]. Microwave drying has a lower drying period, high-energy efficiency, high-drying rate, a high rehydration capacity compared to the convective drying technique [15]. It causes the material to dry spherically by causing heat and mass transfer from the inside to the outside. However, this method's most remarkable disadvantage is that local burns occur by causing polarization at high power levels, especially in fruits with high sugar content [20, 21].
When the literature was examined, it was seen that there were a few studies in which the orange fruit was dried using the convective drying method; however, the microwave drying method was not used directly [22]. In some studies, low doses of microwave output powers were combined with convective drying [23], or the microwave method was applied as a pretreatment [13, 24]. In these studies, only the drying kinetics of the dried orange fruit were examined, and its thermal properties, color parameters, browning index, whitening index, and vitamin C contents were not investigated. At this point, our study serves as a guide for future studies.
The aim of this study is to; (i) determine the drying kinetics of orange slices dried by convective and microwave drying methods, (ii) measured the energy consumption of the techniques, (iii) compare fresh and dried orange slices in terms of vitamin C, color parameters, browning and whitening indexes, and (iv) decide the most suitable drying method according to quality and drying parameters.
Material and methods
Material
Orange fruits (Citrus sinensis L. var. “Washington novel”) were purchased from a local grocery store, and healthy fruits of the average size were used in the trials. Fruits were kept in a cooling unit providing humidity control at + 4 °C until the drying process. Orange massing 100 ± 0.10 g was used in each trial, and the fruits were sliced transversely in equal thicknesses of 0.5 ± 0.05 cm with a slicing apparatus. The slices used in the drying trials were cut from the central part of the fruit, corresponding to the pulpy and juicy parts.
Drying processes
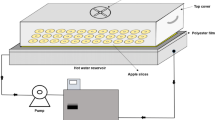
Both microwave and convective drying experiments were carried out using an intermittent microwave dryer (Arçelik, MD 592, Turkey) with operating conditions of 50 Hz, 2900 W, and ~ 10 A. The oven's microwave function can operate at eight different output power ranging from 90 to 1000 W. Convective drying function can be adjusted to nine different temperatures between 50 and 250 °C. Also, an air velocity of 1 m s−1 is produced with the help of a fan mounted in the middle of the oven's inner-rear wall during convective drying. The oven's magnetron, which produces microwave energy, provides energy for 30 s and cuts the energy for the next 30 s. Thus, the intermittent function is completed. Simultaneously, in cases where the microwave energy is activated or passivized, the 280 cm glass rotary table, where the products are placed in the oven, changes the direction of rotation to the right or left. Thus, more uniform heating is provided on the surfaces of the products. Microwave output powers of 90, 160, 350, 500, 650, 750, 850, and 1000 W were used during the microwave drying, while convective drying trials were fulfilled at 50, 75, 100, and 125 °C along with 1 m s−1 air velocity. The mass loss due to time in the dried product was measured by a system that automatically saves the results obtained from a precision scale attached to the bottom of the glass rotary table where the products are placed. By using the initial and final masses of the products kept in an oven at 105 °C for 24 h, the initial moisture content was determined using the following equation:
where Mo is the initial moisture content of orange slices (kg water kg−1dry matter), Wwater is the water mass of the product (g), and WDM is the dry mass of the product (g).
The drying rate (DR) and moisture content (MR) were, respectively, calculated using the following equations:
where DR is the drying rate (kg water kg −1DM min−1), Mt + dt is the moisture content at t + dt time (kg water kg −1DM ), Mt is the moisture content at t time (kg water kg −1DM ), dt is the drying time at t time (min), MR is the moisture content, M is the moisture content at any time (kg water kg −1DM ), Mo is the initial moisture content of the material (kg water kg −1DM ), and Me is the equilibrium moisture content (kg water kg −1DM ). Since the drying time is short and drying is provided under controlled conditions, Me is accepted as zero [25].
In each drying experiment, the energy consumption was determined using a single-phase electricity meter (Makel, M600 2251, Turkey) connected directly to the dryer [26].
Effective moisture diffusivity and activation energy
Experimental data was explained by Fick’s diffusion equation [27]. Fick’s second law of unsteady-state diffusion is shown in Eq. (4).
For extended drying times, Eq. (4) was rearranged, replacing "n" with 1.
Diffusivities of orange slices were found by plotting experimental data regarding ln(MR) versus drying time t in Eq. (6) since the plot gave a straight line with a slope as the following equation:
As the temperature is not a measurable variable in microwave drying, the Arrhenius equation was rearranged to determine the relationship between the kinetic rate constant and the microwave output power ratio to sample amount instead of the temperature for calculation of the activation energy. In convective drying, since the temperature is a measurable parameter, the absolute temperature was used instead of microwave output power in the equation. The activation energy for microwave drying was calculated using Eq. (8) and Eq. (9), while for convective drying, it was determined using Eqs. (10) and (11) [28, 29].
where k is the drying rate constant obtained by using the modified Henderson and Pabis' equation (min−1), ko is the pre-exponential constant (min−1), Deff is the effective moisture diffusivity (m2 min−1), Do is the pre-exponential factor (m2 min−1), Ea is the activation energy (W g−1), P is the microwave output power (W), and m is the mass of fresh material (g).
where Ea is the activation energy (kJ mol−1), T is the absolute temperature (K), and R is the universal gas constant (8.314 × 10–3 kJ mol−1 K−1).
The relationship between the theoretical drying rate constant (kth) and the theoretical effective moisture diffusivity was shown in the following equation:
where kth is the theoretical drying rate constant (min−1), (Deff)th is the effective theoretical diffusivity (m2 s−1), and A is the stabilization constant (s min−1 m−2).
Determined of thermal properties
Some thermal properties of orange slices, namely specific heat, thermal conductivity, thermal diffusivity, and thermal effusivity, as a function of moisture content according to the dry base, were determined via calculation. Specific heat was calculated using the following equation (Eq. 13) [30]:
where Cp is the specific heat (J kg-1 K-1), and X is the moisture content as the dry base at any time (kg water kg −1DM ).
The thermal conductivity of orange slices in microwave and convective drying was determined by the following equation (Eq. 14) [31].
where k is the thermal conductivity (W m−1 K−1).
In calculating the thermal diffusivity of the drying material, it is important to determine the drying material's density primarily. Density was detected by the following equation (Eq. 15) [32, 33]:
where ρ is the density (kg m−3), and X0 is the initial moisture content (kg water kg −1DM ).
Equation 16 shows thermal diffusivity [31, 34], while Eq. 17 used in calculation thermal effusivity [35, 36].
where α is the thermal diffusivity (m2 s−1), and e is the thermal effusivity (W s1/2 m−2 K−1).
Color parameters, browning index, and whitening index
Color measurements of orange slices were carried out using an automatically calibrated colorimeter (Konika Minolta CR10, Japan), measuring according to the CIE Lab method [37]. Accordingly, L, which refers to brightness, a, which is represented redness, and b, which is attributed to yellowness, were measured using the colorimeter; however, C, namely Chroma, and α°, called hue angle, were calculated using the following equations through a and b.
However, the total color change (ΔE) was calculated using the following equation. As the numerical value of the total color change increases, the change in color compared to the fresh product also increases.
where ΔE is the total color change; Lf, af, and bf are the brightness, redness, and yellowness of the fresh product, Ld, ad, and bd are the brightness, redness, and yellowness of the dried ones, respectively.
Another indicator showing the change in color is the browning index (BI) and is calculated using the equations below. As the numerical value of the browning index increases, the darkening that occurs in the product also increases [38].
One of the markers representing the darkening of the product is also the whitening index (WI), and it was calculated with the following equation depending on L, a, and b [39].
Determination of vitamin C
Vitamin C was analyzed according to the method outlined in our previous study [26]. The analysis was performed with a high-performance liquid chromatographic HPLC (PerkinElmer, Waltham, Massachusetts, U.S) method and a C18 SDS column (PerkinElmer, Waltham, Massachusetts, U.S).
Data analysis
The averages of drying data and the standard errors of the estimate along with ANOVA tests were analyzed using the JMP 7 statistical program. All drying trials were performed with three replications and color parameters with twenty-one replications.
Time-dependent moisture ratios (MR) obtained from drying trials were modeled with NLREG2.0 statistical program using 21 different thin-layer drying equations (Eqs. 24–44) defined in Table 1. The same statistical program calculated drying constants and coefficients in the thin layer drying equations. The equation with the closest results to the experimental data was chosen as the most successful drying model. The same statistical program determined the regression coefficient (R2) and the standard error of the estimate (SEE) between the measured and estimated data using thin-layer drying equations. However, the root-mean-square error (RMSE) and Chi-square (χ2) were calculated using the following equations:
where RMSE is the root-mean-square error, MRexp, i is the experimental moisture ratio found in any measurement, MRpre,i is the predicted moisture ratio for this measurement, N is the total number of observations, and ni is the number of constants.
The regression coefficient (R2), the standard error of the estimate (SEE), the root-mean-square error (RMSE), and Chi-square (χ2) were taken as the basis for the selection of the most successful model. Regression coefficient (R2), which should be as close to 1.00 as possible in order to define the model as successful, was the main criterion that enabled a rough selection among thin-layer drying equations. For this reason, the highest R2 was a very important guide in choosing the best model. When the R2 of two or more of the thin layer drying equations were the same, SEE, RMSE, and χ2 were used to determine the most successful model, respectively. The second criterion for the selection of the most successful model was the proximity of SEE, RMSE, and χ2 to 0.00. When similar R2 values were detected, the proximity of these three statistical indicators to 0.00 was examined, respectively. Consequently, the equation or equations that give the closest results to the experimental data are defined as the most successful model.
The parameter k of modified Henderson and Pabis' equation along with ko, Do, and Eo parameters were calculated through the same statistical program, which automatically calculated the coefficient of regression (R2) and the standard error of the estimate (SEE).
Results and discussion
Drying process
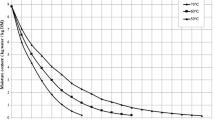
The drying time-dependent moisture content of the orange slices is presented in Fig. 1. Accordingly, orange slices with initial moisture of 6.97 ± 0.02 kg water kg −1DM were dried by convective drying at 50, 75, 100, and 125 °C and microwave drying at 90, 160, 350, 500, 650, 750, 850, and 1000 W until their final moisture was 0.12 ± 0.01 kg water kg −1DM . While the most extended method was convective drying at 50 °C, the shortest one was microwave drying at 1000 W, the highest microwave output power. In microwave drying, the drying time decreased significantly as the microwave output power increased. However, increasing the drying temperature caused a significant shortening of the drying time in convective drying. Drying time at 1000 W, the shortest drying method, was 8.5 times shorter than 90 W with the longest drying time for the microwave drying technique, while 195 times shorter than 50 °C. Convective drying at 50 °C was about 23 times longer than 90 W, the longest-lasting microwave drying technique. The 125 °C dried the orange slices approximately 7 times faster than 50 °C and nearly 3 and 29 times slower than 90 W and 1000 W. Shu et al. [40] dehydrated citrus fruit by microwave drying and determined drying times at 800, 700, and 600 W to be 18, 36, and 38 min, respectively. Their measurements of drying times were in parallel with our study. Talens et al. [19] emphasized that the drying time of orange peels dried with the microwave technique decreases with the increase of the applied power density. Darvishi et al. [41] assigned that by drying lemon slices at 720 W instead of 180 W, there was an 83% reduction in the drying period.
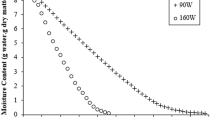
Figure 2 represents the drying rate dependent on moisture content. According to the figure, the average drying rate increased with the increase in microwave power output power, in other words, with the decrease in drying time. Also, the average drying rate increased with the rise in ambient temperature in convective drying. Accordingly, the average drying rate determined at 1000 W was approximately 5 times higher than at 90 W, while it was 334 times higher than at 50 °C. However, the average drying rate at 1000 W was 57, 40, and 16% higher than at 650, 750, and 850 W, respectively. The average drying rate increased 3.38 times as the temperature increased from 50 to 125 °C. Since the drying time was much shorter at microwave output powers between 1000 and 500 W, the constant-rate drying period was not observed; that is, the drying process took place in the falling-rate drying period from the beginning to the end. On the other hand, a constant-rate drying period occurred at the end of the drying periods at microwave powers between 350 and 90 W. Also, the constant-rate drying period was monitored at the end of the convective drying processes. Li et al. [15] underlined that the drying rate in convective drying increases with the increase of the applied temperature and the shortening of the drying time. Shu et al. [40] reported that the average drying rate of citrus peel increased with increasing microwave power. Similarly, Darvishi et al. [41] keynoted that the increase in the microwave power density applied to the lemon slices caused the drying rate to increase significantly.
Modeling of drying data
Figure 3 highlights measured and predicted moisture ratios corresponding to drying time. In the figure, predictions of the most successful models are used in determining predicted moisture ratios. Accordingly, in the 8th minute, corresponding to half of the total drying time at 1000 W, 88% of the total separable moisture evaporated from the orange slices via mass transfer. At 850, 750, 650, 500, 350, 160, and 90 W during the 8th minute of drying, this value was about 84, 79, 74, 68, 55, 44, and 34%, respectively. In convective drying processes at 50, 75, 100, and 125 °C, approximately 88% of the total separable moisture evaporated from the orange slices at 540, 210, 135, and 90 min, respectively. Shu et al. [40] reported that during the sixth minute of the drying period, during the drying of citrus peel at 800 W, 84% of the detachable moisture evaporated from the product, whereas at 500 W, only 48% of the moisture evaporated during the same period. Darvishi et al. [41] stated that during the drying of lemon slices at 720 W, 92.5% of the detachable moisture evaporated from the slices in the second minute of drying, but 60% of the moisture was removed from the product in a similar time at 180 W.
Table 2 shows the regression coefficient (R2), the standard error of the estimate (SEE), the root-mean-square error (RMSE), and Chi-square (χ2) between the experimental data and the predicted data obtained with thin-layer drying equations. According to the table, the experimental data's closest estimates were obtained with the Modified Henderson and Pabis equation in all output powers of microwave drying and all ambient temperatures of convective drying.
Table 3 represents statistical results and coefficients obtained from the Modified Henderson-Pabis thin-layer drying equation for the different microwave power densities. All microwave power densities' regression coefficients were ranged from 0.9997 and 1.000. However, it was ranged from 0.9980 and 0.9999 at convective drying. As both the microwave power density and ambient temperature decreased, the numerical value of k, called drying constant, declined, as well.
Soysal [22] found that k, the drying constant, increased with the increase of microwave output power, just like our findings. Similarly, Alibas [42] emphasized that the k coefficient increased depending on the rising microwave output power.
Effective moisture diffusivities and activation energy
Effective moisture diffusivities and regression coefficients (R2) determined for both microwave and convective drying methods are shown in Table 4. Effective moisture diffusivities in convective drying were calculated according to temperature and the universal gas constant using the Arrhenius equation. However, the equation was rearranged by considering microwave output power and product mass instead of temperature and the universal gas constant in microwave drying. Accordingly, the increase in both microwave output power and ambient temperature led to an increase in effective moisture diffusion. Similarly, the increase in not only the microwave output power but also ambient temperature caused also the slope to increase. The slope was 0.027 at 90 W and 0.997 at 1000 W, while effective moisture diffusivity was 5.46 × 10–11 m2 s−1 at 90 W and 2.04 × 10–09 m2 s−1 at 1000 W. Effective moisture diffusivities' regression coefficients obtained from experimental data at microwave output powers between 90 and 1000 W varied between 0.9504 and 0.9625. Slope and effective moisture diffusivity at 125 °C were, respectively, 0.0123 and 2.2713 10–10 m2 s−1, while these values were, respectively, 0.0049 and 2.7023 10–9 m2 s−1 at 50 °C. The regression coefficients of effective moisture diffusivities in convective drying were between 0.9467 and 0.9769.
Figure 4 represents the relationship between kinetic rate constant, k, for the Modified Henderson and Pabis model and sample amount/output power for microwave drying, whereas Fig. 5 points out the association with effective moisture diffusivities, Deff, versus sample amount/output power for the same drying method. Both figures, in which nonlinear equations were placed, had a similar trend to each other. The activation energy (Ea) of orange slices dried with microwave technique was calculated through these nonlinear equations derived from the relationships not only between k and m/P but also between Deff and m/P. According to these equations, the activation energy calculated in Fig. 4 was 60.36 W g−1, but in Fig. 5 was 60.80 W g−1. Both activation energies calculated through these two equations were almost the same as each other. The regression coefficient (R2) of the relationship between k and m/P in Fig. 4 was 0.9539. In contrast, the regression coefficient (R2) of the relationship between Deff and m/P in Fig. 5 was 0.9818.
Figure 6 shows the relationship between effective moisture diffusivity (Deff) and 1/RT, defined as the ratio of temperature and the universal gas constant to 1. On the other hand, Fig. 7 represents the relationship between the drying constant k and 1/RT of the modified Henderson and Pabis equation, which is the ideal model in convective drying. Just as the related figures in microwave drying, Figs. 6 and 7 had a similar trend. The activation energy was calculated according to the relationship between Deff and 1/RT is 20.60 kJ mol−1. Contrarily, it was determined in the relationship between k and 1/RT was 20.65 kJ mol−1. The regression coefficient of the relationship between Deff and 1/RT was 0.9922, but that of the association between k and 1/RT was 0.9584.
Figure 8 addresses the linear relationship between the theoretical kinetic rate constant (kth) of the Modified Henderson and Pabis equation and the effective theoretical moisture diffusivities ((Deff)th) in microwave drying. At the same time, the figure represents the equation and regression coefficient of this linear relationship. Accordingly, the regression coefficient of the linear relationship between kth and (Deff)th was 0.9972. The value of the stabilization constant (A) was 1.0831 1008 s min−1 m−2.
Figure 9 represents the linear association between the kth of Modified Henderson and Pabis equation, called the ideal model in convective drying, and Deff(th). The linear regression coefficient of this relationship was 0.9993. Also, the stabilization coefficient (A) in convective drying was also calculated as 3.0946 1007 s min−1 m−2.
Rafiee et al. [22] examined the drying kinetics of oranges by convection drying at 40, 50, 60, 70, and 80 °C, and determined that the effective diffusivity increase with the rise of temperature. Also, they calculated the activation energy in convective drying to be 18 kJ mol-1, which was parallel to our study. Deng et al. [2] stated that the effective diffusivity of orange peels decreased due to the fall in temperature in convective drying. Darvishi et al. [41] emphasized that the effective diffusivity of lemon slices dried with microwave technique increases with increasing output power. They reported that the activation energy in microwave drying was approximately 5.6 times lower than ours at 10.911 W g-1. Deng et al. [2] Just as in our work, Dadali et al. [43] also found that effective moisture diffusivity of spinach leaves dried with microwave drying increases with the increase in microwave output power. Similarly, Evin [44] reported that increasing microwave output power also increased effective moisture diffusivity of Gundelia tournefortii L. dehydrated by microwave technique. Al-Harahsheh et al. [45] addressed that the effective moisture diffusivity between 160 and 800 W varied from 1.14 × 10–06 to 6.09 × 10–06 m2 s−1. Sarimeseli [46] highlighted that the effective moisture diffusivities of microwave dried coriander leaves between 180 and 900 W output powers were varied from 0.63 × 1010 to 2.20 × 1010 m2 s−1. Besides, they stated that the slope also increased with increasing microwave power. Both Demirhan and Ozbek [47] and Alibas and Kacar [27] found that the activation energy of celery leaves calculated not only between k and m/P but also between Deff and m/P was similar to each other.
Energy consumption
Table 5 figures out the drying time and average drying rate as well as the energy consumption values of the drying methods. According to the table, energy consumption peaked at 650 W with a value of 0.325 kWh for microwave drying. At microwave output powers below and above 650 W, where the peak was measured, energy consumption decreased with the increase or decrease in output power. The energy consumption measured in microwave drying at 650 W was 21.72% and 59.31% higher than at 1000 W and 90 W, respectively. In contrast, the energy consumption measured in the convective drying method was much higher than that recorded at all microwave output powers. The energy consumption measured at 50 °C was approximately 280, 446, 4, and 5 times higher than at 650 W, 90 W, 75 °C, and 125 °C, respectively.
Thermal properties
Figure 10 sheds light on the time-dependent specific heat and thermal conductivity of orange slices dried by microwave and convective drying methods. On the other hand, Fig. 11 focuses on the time-dependent thermal diffusivity and thermal effusivity of the samples dried by microwave and convective drying methods. According to both figures, all of these four thermal properties were maximally measured in fresh oranges, the highest moisture content, but tended to decrease as the product dried. Except for thermal diffusivity, all other thermal properties of the completely dried products were detected to be at the lowest level. The main reason for the high specific heat of the fresh product, which is approximately 85% water, is due to the natural phenomenon, which includes the high specific heat of the water to prevent the rapid cooling and heating of living metabolisms in nature. Therefore, the specific heat also decreased due to the loss of water in the product during drying [48, 49]. Moreover, if the water, which is high in the pores of the fresh products, is replaced by air via drying, that is, by evaporation, the specific heat of the dry samples decreases [50]. In a striking view, the increase in the specific heat of the raw material with the increase in moisture content can also be due to the adhesion force of the high water rate in the fresh product content [51].
On the other hand, the reason for the high thermal conductivity is that fresh products with high water content have a lot of water in their tissues but lack air. In dried products, the air takes up the place of the tissues emptied by the evaporation of water by drying, and therefore thermal conductivity increases [52]. From another perspective, due to the high number of ions and dipoles in fresh samples with high moisture content, the lattice vibration increases, and this ensures high thermal conductivity [53]. The reason for being high the thermal diffusivity and thermal effusivity of fresh samples is that the thermal conductivity increase rate is higher than the specific heat and bulk density at the high moisture content [53].
The mean specific heat of fresh orange slices was 3764.79 ± 0.38 J kg−1 K−1. The average specific heat decreased as the microwave output power decreased. While the average specific heat was 2404.39 J kg−1 K−1 at 1000 W, the highest power level, this value was 2080.44 J kg−1 K−1 at the lowest power of 90 W. Similarly, we observed that the average specific heat increases with the increase of temperature in convective drying. The average specific heat, which was 1946.81 J kg−1 K−1 at 50 °C, was 2466.54 J kg−1 K−1 at 125 °C. However, the specific heat of orange slices dried by both convective and microwave drying varied between 1190.65 and 1192.92 J kg−1 K−1.
The thermal conductivity, thermal diffusivity, and thermal effusivity of fresh orange slices are 0.385 W m−1 K−1, 1.22 × 10–07 m2 s−1, and 1103.45 W s1/2 m−2 K−1, respectively. Just like the average specific heat, the average values of thermal conductivity, thermal diffusivity, and thermal effusivity also increased with increasing power in microwave drying and with increasing temperature in convective drying. The average thermal conductivity, which is 0.162 and 0.158 W m−1 K−1 at 1000 W and 125 °C, respectively, was calculated as 0.128 and 0.116 W m−1 K−1 at 90 W and 50 °C. The thermal conductivity of microwave and convective dried products had a value of 0.0606 W m−1 K−1.
Since the final moisture contents of the dried orange slices were the same, the density, which is determined as 839.41 kg m−3 in fresh product and used in the calculation of thermal diffusivity and effusivity, was similar with 693.98 kg m−3 in all-drying methods.
The average thermal diffusivity and average thermal effusivity were found as 8.24 × 10–08 m2 s−1 and 535.50 W s1/2 m−2 K−1 at 1000 W, respectively; however, these values are 8.18 × 10–08 m2 s−1 and 532.06 W s1/2 m−2 K−1 at 125 °C. While the average thermal diffusivity was determined as 7.85 × 10–08 and 7.66 × 10–08 m2 s−1 at 90 W and 50 °C, the average thermal effusivity at the same drying levels was calculated as 437.51 and 401.17 W s1/2 m−2 K−1. Since the final moisture level is almost the same in all drying methods, the thermal diffusivity of dry products is also almost the same in each method with 7.33 × 10–08 m2 s−1. The thermal effusivity of the products dried by different techniques varied between 223.57 and 224.08 W s1/2 m−2 K−1. Similarly, Ajala et al. [54] calculated that as the temperature used in the study increased, the thermal diffusivity decreased. Septien et al. [55] underlined that while the thermal diffusivity was 14.6 × 10–08 m2 s−1 for the raw material, it declined to 27.0 × 10–08 m2 s−1 in the dried product. Chakraborty et al. [56] determined that as the drying progresses and the moisture content decreases accordingly, the specific heat capacity, thermal conductivity, and thermal diffusivity decrease significantly. Lemus-Mondaca et al. [35] stated that specific heat, thermal conductivity, thermal diffusivity, and thermal effusivity were the highest at fresh Stevia leaves, followed by infrared, vacuum, and convective dried samples.
Color parameters, browning and whitening index
Table 6 presents the color parameters and browning index of orange slices dried by both convective and microwave drying methods compared to fresh produce. According to the table, the closest color parameters to fresh orange slices were measured in the products dried with the microwave drying method at 350 W, 500 W, and 160 W, respectively. Compared to fresh produce, the most color loss occurred with convective drying at 125 °C, 100 °C, and 50 °C. The reason for this dramatic loss of color parameters in convective drying at 50 °C was that more oxidation occurred in the product due to the too-long drying time. On the other hand, the loss of color at 125 °C and 100 °C, defined as the highest ambient temperatures, was due to the overheating of the orange slices, which have a heat-sensitive structure. Similarly, the color loss was relatively high at 1000 W, the shortest drying method. The loss of color parameters in the shortest drying technique was caused by the penetration of very high microwave energy into the product; in other words, excessive heat increases in the product. Despite the adverse effects of 125 °C, 100 °C, and 50 °C on color parameters, 75 °C was a hopeful method for preserving color.
The color change, browning index, and whitening index, which are essential factors in understanding how much color loss the product is exposed to due to heat, temperature, airflow, etc., showed that the microwave drying technique at 350 W is the most effective method in terms of color preservation. On the other hand, 100 °C, 50 °C, especially 125 °C and 1000 W, caused the most color change, browning index, and whitening index, namely, the color quality of the product decreases.
Although there were limited studies in the literature directly related to oranges' drying, most of these studies were on orange juice and peels. Moreover, studies with other citrus fruits were designed similarly, and these studies focused more on peel and fruit juice than fruit. In a very limited number of studies dealing with the fruit part of both oranges and other citrus fruits, the convective drying method was used rather than the microwave technique. Farahmandfar et al. [57] dried Thomson variety orange peels using different techniques and reported that the brightness, redness, and yellowness of the microwave-dried peels at 600 W and 360 W were close to fresh peels. They also stated that the browning index calculated at 360 W was close to the fresh produce. Bozkir [13] dehydrated the Valencia variety with the convective drying method at 50 °C, 60 °C, and 70 °C and emphasized that the color change (ΔE) decreased due to the increase in temperature. We noticed that the color parameters of fresh orange slices measured in a study conducted by Karabacak et al. [58] were in parallel with our results. Deng et al. [2] dehydrated the orange peels with five ambient temperatures between 50–70 °C and, unlike our finding in our study, found that the most color loss occurred at 65 °C. Garau et al. [59] dried orange peel and pulp by convective drying by applying seven ambient temperatures from 30 °C to 90 °C and found that the least color change for peel and pulp occurred at 40 °C and 50 °C, respectively. They detected moderate discoloration at 70 °C, in contrast to our findings.
Analysis of vitamin C
Table 6 illustrates the vitamin C concentration of orange slices dried by convective and microwave drying methods with fresh ones. The closest drying method to the vitamin C content of fresh orange slices was the microwave technique at 350 W. The convective drying ways at 125 and 100 °C, defined as the highest temperatures in this study, adversely affected the concentration of vitamin C in orange slices, extremely sensitive to heat. Similarly, 1000 W, the shortest drying method that causes high heat, and 50 °C, the most prolonged drying method that causes an increase in oxidation, also negatively affected the orange slices' vitamin C concentration. Accordingly, vitamin C measured at 350 W, despite having the highest concentration, was about 75% less than that of fresh produce. The concentration of vitamin C at 125 °C, 100 °C, 1000 W, and 50 °C was 2.31, 2.19, 2.17, and 2.09 lower than that measured at 350 W, respectively. Strikingly, the fresh product's vitamin C content was approximately four times higher than measured at 125 °C, 100 °C 1000 W, and 90 W. Although convective drying methods caused a decrease in vitamin C' concentration, 75 °C was one of the ideal techniques for preserving the concentration.
Bozkir [13] analyzed the vitamin C content of orange slices dried at 50, 60, and 70 °C along with fresh ones and determined that slices dried at 50 °C and 70 °C had 56% and 79% less vitamin C concentrations, respectively, than fresh ones. Karabacak et al. [58] found that the ascorbic acid content of orange slices dried by vacuum drying was approximately 2–2.5 times reduced compared to fresh ones. Deng et al. [2] measured the vitamin C content of convective dried orange peels at different temperatures from 50 to 70 °C. They noted that products dried at 65 °C had higher vitamin content than those dried at other temperatures. However, they addressed that the vitamin content of those dried at 65 °C was reduced by almost half compared to fresh orange peels.
Correlations of measured data
Table 7 shows positive or negative linear correlations among measured or calculated parameters during microwave and convective drying of orange slices. Accordingly, many negative or positive significant linear correlations were determined across color parameters as expected. The strongest of positive relationships were recorded those between L and a or b, between a and b, between ΔΕ and α°, and between BI and ΔΕ. On the other hand, the strongest negative linear relationships were also found between C and ΔΕ, and BI and between WI and BI. Alibas et al. [37] underlined that there are powerful positive linear relationships between L and a or α°, between a and α°, and between b and C.
Strikingly, we observed significant negative correlations at the medium level between Vitamin C and α or ΔΕ. However, we noticed a moderate positive correlation between Vitamin C and Chroma. This positive relationship proved that with the increase of chroma, which represents the vividness of color at high numerical values, vitamin C in orange slices also increased.
On the other hand, we found that there is a highly strong positive relationship between energy consumption and the drying period. This correlation was related to the longer the drying time, the more energy the dryer consumes.
Conclusion
In this study, orange slices were dried from 6.97 ± 0.02 kgwater kg −1DM initial moisture content to 0.12 ± 0.01 kgwater kg−1DM final moisture content using microwave and convective drying methods. Microwave drying processes, which were completed between 16 and 136 min depending on the output power used in the study, were performed at eight different microwave output power levels between 90 and 1000 W. On the other hand, in convective drying processes completed in 460 to 3120 min, four different drying temperatures of 50, 75, 100, and 125 °C and an air velocity of 1 m s−1 were applied together. The average drying rate was 0.668 kgwater kg −1DM min−1 at 1000 W, while this value was 0.002 kgwater kg −1DM min−1 at 50 °C, respectively. The average drying rate was 0.248 kgwater kg −1DM min−1 at 350 W, the optimum drying method.
Experimental data were converted into predictive data using 21 different thin-layer drying equations. In contrast, the best prediction model for all powers of microwave drying and all ambient temperatures of convective drying was the Modified Henderson and Pabis equation. Also, effective moisture diffusivities and activation energy were calculated in the study. While the activation energy for microwave drying was about 60 W g−1, it was nearly 20.5 kJ mol−1 for convective drying.
The energy consumption of all drying methods during the drying process was measured. It was found that almost all output powers of microwave drying had a very low energy consumption compared to convective drying. Among all microwave output powers, the lowest and highest energy consumptions were measured at 90 W and 650 W, respectively.
Due to the evaporation of the water in the orange slices with drying, all of the thermal properties, namely specific heat, thermal conductivity, thermal diffusivity, and thermal effusivity, as well as the density, decreased.
Color parameters closest to the fresh product were obtained at 350 W microwave drying, followed by 500 W and 160 W. The drying methods that damaged the dried products the most in terms of color were 125 °C, 100 °C, 1000 W, and 50 °C. Similarly, the highest color change and browning index were also recorded in these four drying techniques. In contrast, the lowest whitening index was calculated in these methods.
The closest Vitamin C concentration to the fresh product was measured at 350 W, just as determined in color parameters. Not only 125 and 100 °C, which are the highest ambient temperatures, but also 1000 W, which is the shortest drying method where high heat penetrates the product, negatively affected vitamin C concentration. On the other hand, 50 °C with a very long drying time, which causes high oxidation in orange slices, adversely affected the concentration.
The ideal drying, considering both drying and quality parameters, was achieved at 350 W. Orange slices dried at 125 °C, 100 °C, 100 W, and 50 °C caused adverse effects in terms of color parameters and vitamin C.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Özcan MM, Ghafoor K, Juhaimi FA, Uslu N, Babiker EE, Ahmed IAM, Almusallam IA. Influence of drying techniques on bioactive properties, phenolic compounds and fatty acid compositions of dried lemon and orange peel powders. J Food Sci Technol. 2020. https://doi.org/10.1007/s13197-020-04524-0.
Deng LZ, Mujumdar AS, Yang WX, Zhang Q, Zheng ZA, Wu M, Xiao HW. Hot air impingement drying kinetics and quality attributes of orange peel. J Food Process Preserv. 2019. https://doi.org/10.1111/jfpp.14294.
Barbosa AM, Rocha TAF, Saldarriaga JF, Estiati I, Freire FB, Freire JT. Alternative drying of orange bagasse in vibrofluidized bed for use in combustion. Chem Eng Process Process Intensif. 2020;152:107941.
Oikeh EI, Oviasogie FE, Omoregie ES. Quantitative phytochemical analysis and antimicrobial activities of fresh and dry ethanol extracts of Citrus sinensis (L.) Osbeck (sweet Orange) peels. Clin Phytosci. 2020. https://doi.org/10.1186/s40816-020-00193-w.
Pacheco C, García-Martínez E, Moraga G, Piña J, Nazareno MA, Martínez-Navarrete NM. Development of dried functional foods: stabilization of orange pulp powder by addition of biopolymers. Powder Technol. 2020;362:11–6.
González EA, Nazareno MA. Antiradical action of flavonoid-ascorbate mixtures. LWT Food Sci Technol. 2011. https://doi.org/10.1016/j.lwt.2010.09.017.
Fayaerts AF, Luyten W. Vitamin C as prophylaxis and adjunctive medical treatment for COVID-19? Nutrition. 2020;79–80:110948.
Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017. https://doi.org/10.3390/nu9111211.
Fava F, Zanaroli G, Vannini L, Guerzoni E, Bordoni A, Viaggi D, Robertson J, Waldron K, Bald C, Esturo A, Tueros I, Cebrián M, Sebők A, Kuti T, Broeze J, Macias M, Brendle HG. New advances in the integrated management of food processing by-products in europe: Sustainable exploitation of fruit and cereal processing by-products with the production of new food products (NAMASTE EU). New Biotechnol. 2013;30:647–55.
Zou Z, Xi W, Hu Y, Nie C, Zhou Z. Antioxidant activity of citrus fruits. Food Chem. 2016. https://doi.org/10.1016/j.foodchem.2015.09.072.
O’Shea N, Arendt EK, Gallagher E. Dietary fibre and phyto- chemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov Food Sci Emerg Technol. 2012. https://doi.org/10.1016/j.ifset.2012.06.002.
Fratianni A, Albanese D, Mignogna R, Cinquanta L, Panfili G, Di Matteo M. Degradation of carotenoids in apricot (Prunus armeniaca L.) during drying process. Plant Foods Hum Nutr. 2013. https://doi.org/10.1007/s11130-013-0369-6.
Bozkir H. Effects of hot air, vacuum infrared, and vacuum microwave dryers on the drying kinetics and quality characteristics of orange slices. J Food Process Eng. 2020. https://doi.org/10.1111/jfpe.13485.
Amami E, Khezami L, Vorobiev E, Kechaou N. Effect of pulsed electric field and osmotic dehydration pretreatment on the convective drying of carrot tissue. Drying Technol. 2008. https://doi.org/10.1080/0737393070153729.
Li J, Li Z, Li L, Song C, Raghavan GSV, He F. Microwave drying of balsam pear with online aroma detection and control. J Food Eng. 2021. https://doi.org/10.1016/j.jfoodeng.2020.110139.
Jiang N, Liu C, Li D, Zhang Z, Liu C, Wang D, Niu L, Zhang M. Evaluation of freeze drying combined with microwave vacuum drying for functional okra snacks: antioxidant properties, sensory quality, and energy consumption. LWT Food Sci Technol. 2017. https://doi.org/10.1016/j.lwt.2017.04.015.
An K, Zhao D, Wang Z, Wu J, Xu Y, Xiao G. Comparison of different drying methods on Chinese ginger (Zingiber officinale roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016. https://doi.org/10.1016/j.foodchem.2015.11.033.
Zielinska M, Michalska A. Microwave-assisted drying of blueberry (Vaccinium orymbosum L.) fruits: drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem. 2016. https://doi.org/10.1016/j.foodchem.2016.06.003.
Talens C, Arboleya JC, Castro-Giraldez M, Fito PJ. Effect of microwave power coupled with hot air drying on process efficiency and physico-chemical properties of a new dietary fibre ingredient obtained from orange peel. LWT-Food Sci Technol. 2017. https://doi.org/10.1016/j.lwt.2016.11.036.
Chen A, Achkar GE, Liu B, Bennacer R. Experimental study on moisture kinetics and microstructure evolution in apples during high power microwave drying process. J Food Eng. 2021. https://doi.org/10.1016/j.jfoodeng.2020.110362.
Zielinska M, Sadowski P, Błaszczak W. Freezing/thawing and microwave- assisted drying of blueberries (Vaccinium corymbosum L.). LWT Food Sci Technol. 2015;62(1):555–63.
Rafiee S, Sharifi M, Keyhani A, Omid M, Jafari A, Mohtasebi SS, Mobli H. Modeling effective moisture diffusivity of orange slice (Thompson cv.). Int J Food Prop. 2010. https://doi.org/10.1080/10942910802144345.
Díaz GR, Martínez-Monzó J, Fito P, Chiralt A. Modelling of dehydration-rehydration of orange slices in combined microwaveyair drying. Innov Food Sci Emerg Technol. 2003. https://doi.org/10.1016/S1466-8564(03)00016-X.
Abano E. Microwave and blanching pretreatments for hot air drying of orange-fleshed sweet potato slices (Ipomoea batatas). Int J Food Sci. 2020. https://doi.org/10.1155/2020/8872429.
Soysal Y. Microwave drying characteristics of parsley. Biosyst Eng. 2004. https://doi.org/10.1016/j.biosystemseng.2004.07.008.
Alibas I, Yilmaz A, Asik BB, Erdoğan H. Influence of drying methods on the nutrients, protein content and vitamin profile of basil leaves. J Food Compos Anal. 2021. https://doi.org/10.1016/j.jfca.2020.103758.
Alibas I, Kacar O. Microwave drying kinetics, hypericin content, effective moisture diffusivity and activation energy of Hypericum perforatum L. J Essent Oil Bear Plants. 2016. https://doi.org/10.1080/0972060X.2016.1159530.
Şeremet L, Botez E, Nistor OV, Gogus F, Andronoiu DG, Mocanu GD. Influence of drying conditions on the effective diffusivity and activation energy during convective air and vacuum drying of pumpkin. The Ann Univ Dunarea de Jos of Galati Fascicle VI Food Technol. 2015;39(2):20–9.
Komolafe CA, Oluwaleye IO, Adejumo AOD, Waheed MA, Kuye SI. Determination of moisture diffusivity and activation energy in the convective drying of fish. Int J Heat Technol. 2018; https://doi.org/10.18280/ijht.360414
Huang S, Yang J, Lee Y. Interactions of heat and mass transfer in steam reheating of starchy foods. J Food Eng. 2013. https://doi.org/10.1016/j.jfoodeng.2012.08.007.
Ruiz-López II, Córdova AV, Rodríguez-Jimenez GC, García-Alvarado MA. Moisture and temperature evolution during food drying: effect of variable properties. J Food Eng. 2004. https://doi.org/10.1016/S0260-8774(03)00290-5.
Perussello CA, Kumar C, Castilhos F, Karim MA. Heat and mass transfer modeling of the osmo-convective drying of yacon roots (Smallanthus sonchifolius). Appl Therm Eng. 2014. https://doi.org/10.1016/j.applthermaleng.2013.10.020.
Tzempelikos DA, Mitrakos D, Vouros AP, Bardakas AV, Filios AE, Margaris DP. Numerical modeling of heat and mass transfer during convective drying of cylindrical quince slices. J Food Eng. 2015. https://doi.org/10.1016/j.jfoodeng.2015.01.017.
Koua BK, Koffi PME, Gbaha P. Evolution of shrinkage, real density, porosity, heat and mass transfer coefficients during indirect solar drying of cocoa beans. J Saudi Soc Agric Sci. 2019. https://doi.org/10.1016/j.jssas.2017.01.002.
Lemus-Mondaca R, Zura-Bravo L, Ah-Hen K, Di Scala K. Effect of drying methods on drying kinetics, energy features, thermophysical and microstructural properties of Stevia rebaudiana leaves. J Sci Food Agric. 2021. https://doi.org/10.1002/jsfa.11320.
Jayalakshmy MS, Philip J. Thermophysical properties of plant leaves and their influence on the environment temperature. Int J Thermophys. 2010. https://doi.org/10.1007/s10765-010-0877-7.
Alibas I, Zia MP, Yilmaz A, Asik BB. Drying kinetics and quality characteristics of green apple peel (Mallus communisL. Var. “Granny Smith”) used in herbal tea production. J Food Process Preserv. 2020. https://doi.org/10.1111/jfpp.14332.
Maskan M. Kinetics of colour change of kiwifruits during hot air and microwave drying. J Food Eng. 2001. https://doi.org/10.1016/S0260-8774(00)00154-0.
Cuccurullo G, Giordano L, Metallo A, Cinquanta L. Drying rate control in microwave assisted processing of sliced apples. Biosyst Eng. 2018. https://doi.org/10.1016/j.biosystemseng.2018.03.010.
Shu B, Wu G, Wang Z, Wang J, Huang F, Dong L, Zhang R, Wang Yu, Su D. The effect of microwave vacuum drying process on citrus: drying kinetics, physicochemical composition and antioxidant activity of dried citrus (Citrus reticulata Blanco) peel. J Food Meas Charact. 2020; https://doi.org/10.1007/s11694-020-00492-3
Darvishi H, Khoshtaghaza MH, Minaei S. Drying kinetics and colour change of lemon slices. Int Agrophys. 2014. https://doi.org/10.2478/intag-2013-0021.
Alibas I. Energy consumption and colour characteristics of nettle leaves during microwave. Vacuum Convect Dry Biosyst Eng. 2007. https://doi.org/10.1016/j.biosystemseng.2006.12.011.
Dadali G, Demirhan E, Ozbek B. Microwave heat treatment of spinach: drying kinetics and effective moisture diffusivity. Dry Technol. 2007. https://doi.org/10.1080/07373930701590954.
Evin D. Thin layer drying kinetics of Gundelia tournefortii L. Food Bioprod Process. 2012. https://doi.org/10.1016/j.fbp.2011.07.002.
Al-Harahsheh M, Al-Muhtaseb AH, Magee TRA. Microwave drying kinetics of tomato pomace: effect of osmotic dehydration. Chem Eng Process Process Intensif. 2009. https://doi.org/10.1016/j.cep.2008.06.010.
Sarimeseli A. Microwave drying characteristics of coriander (Coriandrum sativum L.) leaves. Energy Convers Manage. 2011. https://doi.org/10.1016/j.enconman.2010.10.007.
Demirhan E, Ozbek B. Thin-layer drying characteristics and modeling of celery leaves undergoing microwave treatment. Chem Eng Commun. 2011. https://doi.org/10.1080/00986445.2011.545298.
Ikegwu OJ, Ezeh CQ. Thermal properties of Kerstingiella geocarpa seeds as influenced by moisture content. Niger Food J. 2012;30(2):100–5.
Zielinska M, Ropelewska E, Zapotoczny P. Effects of freezing and hot air drying on the physical, morphological and thermal properties of cranberries (Vaccinium macrocarpon). Food Bioprod Process. 2018. https://doi.org/10.1016/j.fbp.2018.04.006.
Mvondo RRN, Damfeu JC, Meukam P, Jannot Y. Influence of moisture content on the thermophysical properties of tropical wood species. Heat Mass Transf. 2020. https://doi.org/10.1007/s00231-019-02795-8.
Alnefaie KA, Abu-Hamdeh NH. Specific Heat and Volumetric Heat Capacity of Some Saudian Soils as affected by Moisture and Density. Appl Mech Mater. 2014; https://doi.org/10.4028/www.scientific.net/AMM.575.103
Cosenza P, Guérin R, Tabbagh A. Relationship between thermal conductivity and water content of soils using numerical modelling. Eur J Soil Sci. 2003;54:581–7.
Yu DU, Shrestha OD, Baik OD. Thermal conductivity, specific heat, thermal diffusivity, and emissivity of stored canola seeds with their temperature and moisture content. J Food Eng 2015; http://dx.do.org/https://doi.org/10.1016/j.foodeng.2015.05.12
Ajala AS, Ogunsola AD, Odudele FB. Evaluation of drying temperature on proximate, thermal and physical properties of cocoyam flour. GJEDT. 2014;3(4):13–8.
Semptien S, Mirara SW, Makununika BSN, Singh A, Pocock J, Velkushanova K, Buckley CA. Effect of drying on the physical and chemical properties of faecal sludge for its reuse. J Environ Chem Eng. 2020. https://doi.org/10.1016/j.jece.2019.103652.
Chakraborty S, Mazumder S, Soumitra B. Changes In Thermal Properties And Colour Attributes Of Potato (Chandramukhi Variety) During Foam Mat And Thin Layer Drying. JMBFS. 2017; https://doi.org/10.15414/jmbfs.2017.6.5.1121-1126
Farahmandfar R, Tirgarian B, Dehghan B, Nemati A. Changes in chemical composition and biological activity of essential oil from Thomson navel orange (Citrus sinensis L. Osbeck) peel under freezing, convective, vacuum, and microwave drying methods. Food Sci Nutr. 2020. https://doi.org/10.1002/fsn3.1279.
Karabacak ÖA, Acaroğlu A, Ömeroğlu RY, Çopur ÖU. Microwave pre-treatment for vacuum drying of orange slices: drying characteristics, rehydration capacity and quality properties. J Food Process Eng. 2020. https://doi.org/10.1111/jfpe.13511.
Garau MC, Simal S, Rosselló C, Femenia A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007. https://doi.org/10.1016/j.foodchem.2007.01.009.
Acknowledgements
We thank Mahrukh Parveez Zia, whose main speaker in English, is to help us edit the manuscript in terms of language.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Ilknur Alibas: supervisor, designing the study, modeling data, data statistics, calculating effective moisture diffusivity and activation energy, quality parameters analyses such as color, vitamin C, browning, and whitening index, writing, and reviewing the manuscript. Aslihan Yilmaz: drying the material, modeling data, data statistics, investigation, and analyses of color parameters.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Consent to participate
The study does not involve any animal and human component dealing with genetically manipulated materials either in the process or experiment.
Ethical approval
Ethics approval is not required, as the study does not include any animal or human stuff.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alibas, I., Yilmaz, A. Microwave and convective drying kinetics and thermal properties of orange slices and effect of drying on some phytochemical parameters. J Therm Anal Calorim 147, 8301–8321 (2022). https://doi.org/10.1007/s10973-021-11108-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11108-3