Abstract
The paper presents results concerning thermal decomposition of copper II salts (acetylacetonate, acetate monohydrate and nitrate trihydrate) in synthetic air and argon flow. Thermogravimetry tests coupled with mass spectrometry were performed in temperature range of 25–1300 °C. The influence of salt addition on the rheological properties and sintering behaviour of ceramic samples was then investigated. The microstructure evolution based on light and scanning electron microscopies coupled with stereological methods was described. The performed investigations revealed not only the differences in thermal decomposition of examined salts but also differences in the phenomena related to the oxidation and reduction processes of copper products. The atmosphere has also influenced the mechanisms of salts decomposition as well as further changes in decomposition products. Moreover, the presence of ZnO powder has changed the thermal decomposition process of copper II acetylacetonate. The investigations showed that acetates and nitrates can be efficient in homogeneous distribution of small amounts of metal additives in the ZnO-based suspensions. Higher concentrations of these type of salts lead to the increase of viscosity due to the fact that acetates and nitrates increase the ionic strength in the slurry. On the other hand, acetylacetonates do not disturb the stability of the slurries. However, they are characterized by low water solubility and thus homogenous distribution of small amount of these dopants is more difficult. The addition of copper increased the grain size of the sintered ZnO samples. The copper was not segregated at grain boundaries in the form of oxide, it diffused into ZnO matrix.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Industrial development as well as higher demands of consumers require continuous progress in materials science. There is a need to invent new materials or modify properties of known one in order to expand their application or obtain devices with long live time and better properties. One of the methods to improve the properties of the materials, also ceramic materials, is to use dopants [1,2,3]. However, doping can be realized at two different stages of element production: during synthesis of the powder or during shaping of the product [4,5,6,7]. The idea of doping ceramic materials (powder or layers) at synthesis stage is usually based on dissolving precursors of major and dope chemical element in a solvent (aqueous or organic) followed by chemical precipitation or gelling (sol–gel method). The last stage is heat treatment in order to remove residuals of the solvent or to obtain desired phase composition, for example calcination to obtain oxide phase from hydroxides. The details of these techniques can be find elsewhere [4, 8, 9]. A good example of modern material which is obtained by doping at the synthesis stage is ATO (antimony-doped tin oxide), belonging to the group of TCO (transparent conductive oxides). Substitution of tin atoms by antimony leads to significant increase in electrical conductivity [10, 11]. The interesting electrical properties exhibit also zinc oxide, which is n-type semiconductor with wide band gap of ~3.3 eV at 300 K and high excitation energy. It has gained more attention as semiconductor material for sensing, electronic and optoelectronic applications [12]. The properties of ZnO can be improved by doping at synthesis stage. For example, Wang et al. [13] revealed that Ni doping of ZnO significantly improved ethyne sensing properties and maximum sensitivity to C2H2 was observed for 5at% addition of Ni. The advantage of the doping at the synthesis stage is the possibility to control the size and shape of the particles or microstructure and thickness of the films. These methods allow also to obtain homogenous distribution of dopant in the crystal structure of the major phase. On the other hand, the morphology of the doped powder is very sensitive to the process parameters, which sometimes makes synthesis difficult. The properties of materials can be also modified during forming process. The example of such approach can be the production process of varistors based on zinc oxide [6, 14], where dopants in the form of oxides (Bi2O3, Cr2O3, Sb2O3 and others) are mixed with ZnO and homogenized during milling followed by granulation and pressing. The advantages of such approach are simplicity of the process and possibility to use powders which are available on the market. However, by this method it is difficult to obtain high homogeneity in the whole volume of the product, long milling time is required. It should be underlined that doping at synthesis stage leads to changes in the crystal structure. Doping at forming stage usually changes the structure of grain boundaries, where additional phases can be formed. Thus, depending on the stage, different properties of the material can be modified.
Metal salts are often used as precursors during doping at synthesis stage. However, they can be also very efficient doping agents during forming, especially when their use is combined with wet forming methods. The advantages of these methods are simplicity, near-net-shaping possibility, they can be used for low and large-scale manufacturing and they don’t require complicated and expensive equipment [15, 16]. Moreover, water can be used as a solvent which fulfil green chemistry approach. The use of water as a solvent is also beneficial in case of using salts as dopants. Salts are a wide group of chemical compounds which are acid–base reaction products, and they are used in wide range of applications. For example, one of interesting application is to use low-temperature eutectic salt for energy storage [17,18,19]. However, salts exhibit different properties since they can be composed of different types of cations and anions. Some are characterized by significant solubility in water and other polar solvents. Thus, in case of wet forming methods, when dopant is in a form of water-soluble salt, it is possible to dispose homogenously in the slurry even its small amount, contrary to mixing of powders. Moreover, by selection of sintering atmosphere (oxidizing, inert or reducing) it is possible to obtain dissimilar phases (metallic or oxide) which are characterized by different properties. This allows to modify the properties of the ceramic materials efficiently. However, in case of higher amounts of metal salt addition, the ionic strength of suspension can be increased which leads to higher viscosity. In wet forming methods, it is crucial to control the properties of the ceramic suspensions, like viscosity, stability and solid loading [20]. Thus, it is important to investigate the influence of different type of salts on rheological properties of the suspensions. The behaviour of metal salts during heat treatment is important for sintering process of ceramic materials. The knowledge about thermally activated transformations which occur in wide temperature range is necessary for proper optimization of sintering conditions. In this case, absolutely essential technique is thermogravimetry coupled with mass spectrometry [21]. The important information from measurements by this technique is the temperature at which decomposition of additives (inorganic or organic) ends and what type of gases are released to the atmosphere during decomposition. Thermal analysis also allows to determine thermal stability of organic and inorganic materials [22, 23]. Thermogravimetry was also applied to investigate thermal behaviour of different metal salts, including copper nitrates and acetates [24,25,26]. However, most of works are focused on the decomposition processes in air (up to 400–500 °C). Such data are insufficient when salts are considered as dopants for ceramics materials. Moreover, the decomposition process of the salt in presence of ceramic powder is also important.
The aim of the work was to investigate the behaviour of copper acetylacetonate, acetate and nitrate during heat treatment in temperature range of 25–1300 °C with the use of thermogravimetry technique coupled and mass spectrometry. Thus, not only decomposition processes of salts were investigated but also following reactions of remained products, which are important in case of using these salts as dopants for ZnO-based ceramic materials. The influence of salts on rheological properties of the ZnO-based suspensions was also analysed. Acetylacetonate, acetate and nitrate have been chosen due to their different solubility in water as well as the presence of different functional groups. All these variables can influence the forming and sintering stages. The impact of copper addition on the microstructure and properties of ZnO was also a subject of this study.
Experimental
Materials
ZnO powder (ZINCO, Chelabinsk) was used in the study. Copper salts, acetylacetonate (synthesized at the Faculty of Chemistry, WUT), acetate, nitrate (Merk) and were chosen as potential dopants for ZnO ceramics (Fig. 1). These salts have different functional group and differ in solubility in water. It should be pointed out that copper nitrate and acetate have been used in a form of trihydrate and monohydrate, respectively.
DTA/TG measurements
DTA/TG measurements were carried out by using Netzsch STA 449C Jupiter coupled with Quadrupole Mass Spectrometer Netzsch QMS 403C. The analyses were performed by using two programs in order to examine the influence of the atmosphere on the type of processes occurring during the decomposition of the samples and the type of released gases. The parameters of these programs are shown in Table 1.
Samples preparation for DTA/TG analysis
Three copper salts: copper (II) acetylacetonate, copper (II) acetate monohydrate, copper (II) nitrate trihydrate and green body specimen composed of ZnO powder and copper (II) acetylacetonate, obtained by slip casting method, have been chosen for the thermal analysis. Mass spectrometer was set to detect m/z values in mass range of 10–300.
The copper salts have been used as received and each of them have been placed in the measuring crucible. The quantities of analysed copper salts equalled ca. 20 mg. These samples have been covered by non-reactive Al2O3 powder (calcinated at 1300 °C for 2 h) in the quantity of 0.3 g in order to prevent the sample creeping from the crucible. In case of ZnO green body, it has been crashed in the mortar before the measurement. The ZnO sample was placed in the crucible without any coverage and the quantity equalled ca. 35 mg. All the analysed samples have been measured by using both measuring programs shown in Table 1.
Suspension preparation and rheological test
ZnO-based aqueous suspension of 45vol% of solid loading was prepared. Poly(acrylic acid)-based polyelectrolyte (PAA) of commercial name DURAMAX™ D-3005 (DOW) was used as dispersing agent (0.4 mass % of active matter in relation to solid content). Suspensions were mixed and homogenized by ball milling (Retsch PM 200) for 1 h with speed of 300 rpm, followed by degassing in a THINKY ARE-250 Mixing and Degassing Machine (Thinky Corporation). Rheological properties of slurries containing ZnO and Cu salts were investigated by using rotational rheometer Kinexus Pro (Malvern Instruments, UK) in a plate–plate geometry with 0.5 mm gap between plates. The viscosities of the suspensions as a function of shear rate were measured in the range from 0.1 to 100 s−1.
Sintering process and microstructure characterization
Based on DTA/TG measurements, the sintering conditions have been established. The samples, after slip casting, were sintered in air by two-step sintering process with peak temperature at 1200 °C (3 min dwell time) and sintering temperature of 1150 °C (90 min dwell time), with heating rate of 5 °C·min−1. Sintered samples were mounted in a polymeric resin followed by mechanical grinding and polishing in order to obtain flat surface. Prior to microscopic observations, samples were thermally etched at temperature of 1100 °C (5 °C·min−1) heating rate and dwell time of 15 min. The microstructure of the samples was investigated by using light microscope Nikon Eclipse LV150N and scanning electron microscope Hitachi SU-8000 equipped with cold field emission gun. The grain size, shape factors as well as grain size distribution were calculated with the use of Nikon NIS Elements software.
XRD measurements
XRD technique was applied to identify heat-treated products. Since the volume of the samples after DTA/TG measurements was too low for XRD analysis, additional heat treatment of larger samples was done. For this purpose, standard furnaces (Carbolite Gero, type RHF 14/3 and Carbolite, type STF 16/75/450) have been used. Heat treatment parameters are given in Table 1. Copper salt has been covered by non-reactive Al2O3 powder, similarly to DTA/TG measurements. After heat treatment, XRD measurements were done with the use of Rigaku MiniFlex II with CuKα radiation (λ = 1,54,178 Å) at voltage of 30 kV and current of 15 mA. The analysis was performed in 20–100° range of 2θ with measurement step of 0.01° (dwell time 1 s). The obtained results were analysed by using MDI JADE 7 (Materials Data, Inc.) software.
Results and discussion
Thermal decomposition of copper (II) acetylacetonate
DTA/TG curves of the thermal decomposition of copper (II) acetylacetonate in the air and argon atmospheres are shown in Fig. 2. The total mass loss was 76.25% in air and 72.86% in argon. From the beginning of the test, a slight mass loss can be observed up to 200 °C which is caused by the evaporation of the moisture from the sample. This was confirmed by MS analysis where small peak of m/z value 18 (ascribed to H2O) was observed (Fig. 2b,c). Above 200 °C, a rapid mass loss was evidenced for both air and argon atmosphere. However, above 250 °C TG curve for C10H14CuO4 decomposition in air became less steep and reached the minimum at 350 °C. In case of decomposition in argon, no changes in slope of TG curve was observed and the mass loss stops at 260 °C. This stage is characterized by mass loss of 79.68% (in air) and 77.06% (in argon) and can be ascribed to the decomposition of copper (II) acetylacetonate. During this stage, the formation of Cu and Cu2O also takes place [27]. An exotherm on DTA curve in air with the maximum at 266.8 °C is attributed to the decomposition of copper II acetylacetonate by removal of coordinating acetylacetone ligands from the central metal atom followed by further decomposition to more simple compounds (CO2). In case of measurement in argon, one endothermic with the minimum at 101.1 °C peak is visible on DTA curve, which is related to the processes of the dehydration of the sample. The exothermic peak with the maximum at 274 °C is also visible. However, this peak is less pronounced compared to the peak on DTA curve obtained in air. Moreover, a small endothermic peak is probably present at 250 °C.
The differences in decomposition of copper II acetylacetonate in air and argon are also visible in MS spectra (Fig. 2b,c). The analysis of MS curves (Fig. 2b) allows to conclude that the main products of thermal decomposition of this salt in air are H2O and CO2. The main m/z values detected by mass spectrometer were 18 and 44 which together with m/z values 17 and 12 can be ascribed to H2O and CO2 molecules, respectively. There is the increase in the intensities of MS 18 and 44 signals with the maxima at 253.4 °C and 263.8 °C what indicates that free acetylacetone ligands are subjected to consecutive decomposition to CO2 and H2O products. The MS signals 41, 42, 43, 45 and 46 are observed with the maximum at 248.2 °C which is lower temperature compared to the temperature of CO2 signal maximum. These signals can be ascribed to hydrocarbon ions, which are formed during the first stage of the thermal decomposition of the acetylacetone ligands [28]. The important information is that intensities of these signals are very low in comparison with signals 18 and 44. The m/z values 41, 42 and 43 may indicate on acetates and acetyl groups what corresponds to the sample structure.
In case of measurements performed in argon, the main gaseous product of the decomposition was H2O which corresponds to m/z 18 (Fig. 2c). The plot of m/z 18 as a function of temperature demonstrates two peaks with maxima at 116.9 °C and 261.2 °C (the highest intensity). Mass spectrometer has also detected m/z values 15, 42, 43 of relatively high intensity which confirms the presence of hydrocarbon ions. Especially interesting is the fact that the high intensity was observed for m/z value 43 which corresponds to acetyl anions or acetone. Thus, the mechanism of decomposition is different in comparison with air atmosphere, where decomposition mostly leads to CO2 formation. The MS 44 signal, which corresponds to CO2 molecule, has lower intensity compared to the measurement carried out in air. Moreover, it seems that the decomposition in argon proceeded in one step while in case of air three steps can be distinguished. The first step is the formation of hydrocarbon ions (probably acetates and acetyl groups). In the second step, formation of hydrocarbon ions proceeds but most of these products decompose to CO2. In this step, copper (II) acetylacetonate decomposes also directly to CO2 and H2O. The peak of m/z 44 (CO2) exhibits a “tail” on the right side (Fig. 2b). This “tail” corresponds to the shape of TG curve (Fig. 2a). Thus, in the third step residuals of the copper II acetylacetonate decomposed only to CO2.
Above 300 °C, the sample exhibited different behaviour depending on the type of the atmosphere. In air, mass increases (10.57%) due to the oxidation of copper and copper (I) oxide and formation of CuO [29]. This step lasts up to 1022 °C and then mass loss step (9.81%) occurs. It overlaps with DTA endothermic peak with the minimum at 1036.3 °C. It should be underlined that the mass balance in these two steps is almost equal to zero (0.76%) which suggests that transformation at 1036.3 °C can be attributed to the decomposition of CuO and repeated formation of Cu2O [30]. The mass increases again (3.42%) in the last fourth step from 1047 °C to the final temperature of 1300 °C.
Analysing the measurement performed in argon only small mass changes are visible after decomposition of copper II acetylacetonate (above 260 °C). The mass change at this stage equals 5.1% (increase) and it results from the oxidation of copper (I) oxide. This effect is lower than for the measurement carried in air. Despite of the presence of inert atmosphere (Ar), small amounts of oxygen can be created from various transformation occurring in the first step of the thermal decomposition. The MS 16 signal confirms the presence of oxygen molecules in the released gases (Fig. 2c).
Thermal decomposition of copper (II) acetate monohydrate
DTA/TG curves of the thermal decomposition of Cu(CH3COO)2 monohydrate in air and argon atmospheres are shown in Fig. 3. Up to 290–300 °C, the TG curves for both atmospheres look similar and display two mass loss steps. It should be pointed out here that mass loss during measurement in air starts at slightly lower temperatures in both, the first and the second stage of decomposition. The first stage with the mass loss of 8.40% for air and 9.27% for argon can be ascribed to the dehydration of analysed samples. They corresponds with endothermic peaks (DTA curves) with the minimum at 131 °C (air) and 105.5 °C (argon). MS analysis revealed peaks of m/z values 17 and 18 (H2O) at 141.7 °C and 148.0 °C for air and argon atmosphere, respectively. Around 240 °C, the second mass loss step occurs which is characterized by the largest mass loss 54.88% (air) and 57.44% (argon). In case of the measurement in air, this mass loss corresponds to MS signals of m/z values 17, 18, 42, 44, 45 (Fig. 3b) of the highest intensity with the maximum at 279.8 °C. Thus, during this stage the decomposition of Cu(CH3COO)2 monohydrate probably leads to acetic acid, acetaldehydes, acetone, H2O and CO2. In the measurement in argon, the presence of additional m/z values (16, 32, 43) was evidenced. MS signal 43 can be ascribed to either propyl or acetic groups while signals 16 and 32 correspond to O2. Thus, decomposition of copper (II) acetate runs also with emission of oxygen which could not be detected during measurement in air, because the presence of air in chamber shields all changes in m/z 16, 32 related to additional O2 appearance. The decomposition process in this stage is associated with the exothermic peaks with the maximum at 286.1 °C (air) and 303.0 °C (argon). Similarly to the decomposition of copper (II) acetylacetonate, a small endothermic peak was observed in this stage during the measurement in argon. The decomposition of copper II acetate leads to the formation of copper and copper (I) oxide [31]. This could explain the differences in mass loss of the sample in this stage between the measurements in air and argon. The presence of oxygen leads to formation of copper (I) oxide while ambient atmosphere leads to formation of metallic copper. Thus, the mass loss is lower in case of the test performed in air.
In copper (II) acetate monohydrate thermal treatment in air, the mass increase step is observed (2.39%) which begins at 297 °C and ends at 1022 °C. It was assigned to the oxidation of Cu and Cu2O to CuO. The mass increase in this step is lower in comparison with the measurement in air for copper II acetylacetonate (10.57%) presented in Fig. 2. This can be explained by the formation of the oxygen molecules during thermal decomposition of copper (II) acetate, which leads to lower concentration of metallic Cu or even formation of CuO phase and, as a result, to further lower oxidation degree and smaller mass increase of samples. In the last step, the mass loss equalled to 3.54% which results from the decomposition of CuO to Cu2O. This phenomenon was also observed in case of thermal decomposition of copper II acetylacetonate. However, the increase and further decrease of mass of the sample related to the oxidation of Cu2O and reduction of CuO are smaller in comparison with C10H16CuO4 sample. It is interesting since the concentration of Cu in copper II acetate monohydrate is higher than in copper II acetylacetonate. Thus, differences in oxidation process can be related to different phase composition (concentrations of Cu and Cu2O) or to different morphology of these phases. The endothermic peak of low intensity is visible on DTA curve during reduction of CuO. In case of the measurement in argon, the transformation from Cu and Cu2O to CuO is almost not visible during thermal decomposition and results from insufficient amount of oxygen in the measuring chamber.
Thermal decomposition of copper (II) nitrate trihydrate
Figure 4a shows DTA/TG curves of thermal decomposition of copper (II) nitrate trihydrate in air and argon atmospheres. In the first stage, the mass loss (39.71%) starts around 103 °C and ends at 225 °C in air atmosphere while in case of test in argon, it occurs between 84 and 208 °C with mass loss 35.42%. In both cases, it can be ascribed to the dehydration process what corresponds to DTA and MS curves. The first stage of dehydration results in the formation of Cu2(OH)3NO3 [32]. However, the mass loss in the first stage is too high for pure dehydration process. The share of water in copper II nitrate trihydrate is equal to 22.4 mass %. Thus, at this stage the beginning of the decomposition of copper II nitrate also takes place. This was confirmed by MS where, besides H2O (m/z values 17.18), signals from nitrogen oxides (m/z value 30) with maxima at 201.5 °C for air and 182.4 °C for argon were also present (Fig. 4b,c). In the second stage, mass loss of 22.87% was observed in the temperature range between 225 and 347 °C (test in air). In case of argon, this step begins at 208 °C and ends at 252 °C with the mass loss 27.81%. At this stage (225–347 °C), successive decomposition of Cu2(OH)3NO3 as well as formation of copper and copper oxide proceeds. On DTA curve, in this temperature range, one exothermic peak with the maximum at 238.3 °C and two endothermic peaks with the minima at 129.4 °C and 181.5 °C are visible which corresponds to the dehydration of Cu(NO3)2·3H2O together with its decomposition. The MS analysis revealed that main gaseous products at this stage are H2O (m/z values 17, 18) and nitrogen oxides (m/z values 30, 46) (Fig. 4b,c). Additionally, the presence of oxygen molecules (m/z value 16, 32) was detected during measurement in argon (Fig. 4c). It is interesting that the temperatures of maxima of oxygen MS signal correspond to maxima of nitrogen oxides. The oxygen is formed also during measurement in air, but it cannot be detected due to the composition of atmosphere as it was explained in the previous section. CO2 is also present in the released gases (m/z 44) for both atmospheres. The investigation revealed that decomposition of the copper II nitrate, contrary to the copper II acetylacetonate and copper II acetate, proceeds in two steps. This is confirmed by two peaks of the m/z signals 30 and 46 with maxima at 201.5 °C and 264.0 °C in case of test in air. For the measurement in argon, the second peak of m/z values 30 and 46 has maximum at 250.8 °C.
In the next stage of the test in air (347–1028 °C), the mass increase (0.26%) is visible and it can be ascribed to the oxidation of Cu and Cu2O to CuO. However, in the comparison with the other copper salts (copper II acetylacetonate and copper II acetate), this mass change was almost negligible. The last stage starts at 1028 °C and can be ascribed to the decomposition of CuO to Cu2O as it was observed in the other salts. However, the mass change associated with this reduction is higher in comparison with the mass increase between 347 and 1028 °C. Thus, it is highly probable that decomposition of copper II nitrate leads directly to formation of CuO, without intermediate phase (Cu and Cu2O). This could be explained by the fact that during decomposition of this salt, the oxygen molecules are formed (Fig. 4c).
In the test in argon, the mass increase after primary salt decomposition has not been detected, resulting of the lack of the oxidation process (Fig. 4c). However, at 850 °C the mass loss over 3.5% was observed. This can be ascribed to the reduction of CuO to Cu2O or Cu since MS analysis revealed m/z value 32 (oxygen molecules) at ca. 928.8 °C. The presence of oxide molecules in this temperature range was not visible in case of other copper salts in argon flow. Thus, only decomposition of copper II nitrate trihydrate in argon leads directly towards copper II oxides as solid products.
Thermal decomposition of copper (II) acetylacetonate in ZnO powder mixture
Figure 5 presents DTA/TG/DTG curves together with mass spectra for the specimen based on ZnO powder and copper (II) acetylacetonate (2 mass % in relation to the mass of the ZnO powder) in air and argon atmospheres. The total mass loss was 2.48% and 7.88% in air and argon, respectively. The total mass loss corresponds mainly to the moisture content and copper salts decomposition in the analysed sample. The difference in total mass loss between samples analysed in air and argon can be attributed to the imperfection of shaping process. The samples based on ZnO powder and copper (II) acetylacetonate for DTA/TG analysis were prepared by milling of samples in a green state obtained by slip casting method. Inhomogeneity of salt distribution in suspension results in different concentration of salt in obtained samples. Thus, the shaping and mixing processes should be improved in order to obtain samples of high homogeneity. In case of analysis in air, the decomposition goes in three steps. The endothermic peak on DTA curve with the minimum at 83.4 °C is visible and can be ascribed to the release of water adsorbed on the surface of the ceramic powder. The biggest mass loss in the first two steps (2.30%) was observed at the temperature range 101–305 °C and corresponds with peaks on the DTG curve. Furthermore, two exothermic peaks on DTA curve with the maxima at 227.5 °C and 278.8 °C are visible. These peaks overlap with the MS 17 and 18 signals with the maxima at 237.9 °C and 232.7 °C, respectively. The other m/z values detected by mass spectrometer have been 12, 43 and 44 with the highest intensities at 232.7 °C and 274.1 °C. These results indicate that the main gaseous products released to the atmosphere during the decomposition of the analysed sample were H2O (MS 17, 18) and CO2 (MS 12, 44). Thus, it allows to assume that decomposition of copper II acetylacetonate in the presence of ZnO is slightly different in comparison with decomposition of pure copper II acetylacetonate. Clear two stages of decomposition can be indicated (Fig. 5b). In the first stage, the major products are H2O, CO2 and acetyl anions or acetone. In the second stage only CO2 and H2O molecules are released. In case of pure copper II acetylacetonate such clear division into two decomposition stages could not be observed. Moreover, in case of the mixture of C10H14CuO4 and ZnO powder, no increase in mass after salts decomposition followed by mass loss at 1036.3 °C was observed, like it was in case of pure copper II acetylacetonate (Fig. 2a). These changes have been related to the oxidation and reduction processes of Cu and copper oxides. However, in Fig. 5a only slow mass loss was observed till the end of the measurement (1300 °C).
The mass loss in the first two steps in argon atmosphere amount to 8.77% at the temperature range 103–850 °C. Up to 250 °C, mass loss is rapid. In this temperature range, two endothermic peaks on DTA curve with the minimum at 105 °C and 249.6 °C are visible. The first peak is related to the processes of dehydration of the sample and the second one can be ascribed to the decomposition of copper (II) acetylacetonate. This peaks overlap with the MS 17, 18 and 12, 44 which can be ascribed to H2O and CO2 molecules. There is the increase in the intensities of MS 18 and 44 signals with the maxima at 245.2 °C and 234.7 °C what indicates the decomposition of copper II acetylacetonate to CO2 and H2O products. The MS signals 42, 43 which are observed with the maximum at 250.4 °C can be ascribed to hydrocarbon ions which are formed during the first stage of the thermal decomposition of the cooper II acetylacetone. Between 250 and 700 °C TG curve became smoother and mass loss was slower. At this temperature rage, MS 44 signal reveals maxima at 297.5 °C and 397.0 °C. In case of decomposition of pure copper (II) acetylacetonate (Fig. 2c), such peaks were not visible but only slightly increase of signal of 44 m/z value. Above 700 °C, TG curve became more steep which corresponds to the exothermic peak with the maximum at 682.7 °C and MS 18, 43, 44 signals. Similarly, but less pronounced, the effect was observed in pure copper (II) acetylacetonate (Fig. 2).
XRD analysis
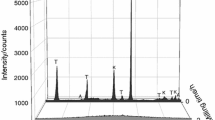
Figure 6 shows XRD spectra of copper II acetylacetonate after heat treatment according to parameters given in Table 1. Analysis evidenced that the main product of heat treatment in air was CuO crystallized in monoclinic crystal system (Fig. 6a). The presence of corundum is due to the fact that calcinated Al2O3 was used to cover salt in the crucible. After annealing, it was difficult to separate degradation products and Al2O3. Thus, the whole powder from the crucible was taken for the analysis. The XRD measurements revealed also that chemical reaction took place between Al2O3 and products of salt degradation (Cu or CuO). This was confirmed by the presence of small peaks which correspond to spinel-type phase CuAl2O4.
Figure 6b shows XRD spectra of copper II acetylacetonate after heat treatment in argon atmosphere. The investigation revealed that the main heat treatment product is metallic Cu of cubic crystal structure. Similarly to the sample annealed in air, the presence of Al2O3 was evidenced. In case of sample annealed in argon, it was difficult to determine other heat treatment products. Only one additional peak (c.a. 31.7 degree of 2θ) was observed. It can be assigned to CuAl2O4, like it was in case of sample annealed in air. However, XRD spectra of sample annealed in argon did not evidence additional peaks of this spinel phase. Thus, it is more likely that this peak corresponds to both (002) and (−111) planes of CuO monoclinic phase [33]. XRD analysis evidenced that by changing the sintering atmosphere it is possible to obtain different heat treatment products of copper II acetylacetonate as well as other salts.
Rheological properties of ZnO suspensions with salts addition
Figure 7 shows viscosity curves of ZnO-based suspensions of 45 mass % of solid loading. Rheological properties were determined for suspensions with and without the addition of copper salts in different forms and concentrations. The concentrations of copper ions in suspension were 0.1 mass %, 0.5 mass % and 2.0 mass %. In order to obtain these values, different quantities of Cu salts were added. For example, concentration of 0.1 mass % Cu ions requires the addition of 0.41 mass % of copper II acetylacetonate or 0.31 mass % of copper II acetate hydrate or 0.38 mass % of copper II nitrate trihydrate. Within the paper, the values of concentrations of Cu ions will be used to describe suspensions, green bodies and sintered samples. The small concentrations of copper (0.1 mass % and 0.5 mass %) in a form of acetylacetonate did not influence one of the rheological properties of the slurries. In these cases, the suspensions exhibited low viscosity which is important during casting process, since it allows to fill the mould properly, even moulds of complicated geometries. The addition of 2 mass % of copper in the form of acetylacetonate increases the viscosity of ZnO-based suspension. This increase could be explained by the fact that solid loading of the suspension is also increased. In order to obtain 2 mass % of copper in the suspension, ca. 8.3 mass % of copper II acetylacetonate should be added. Since this salt is characterized by low solubility in water (0.29 g·100 mL−1), the solid loading of the suspension was increased. It is well known fact that the increase of solid loading leads to higher viscosity of the suspension [34]. On the other hand, small addition of Cu (0.1 mass %) in the form of copper II nitrate increases slightly the viscosity of the suspension. However, the increase of the concentration of Cu to 0.5 mass % (in the form of nitrate) results in the formation of the suspension of high viscosity (paste-like), for which rheological properties have not been measured. The reason of the increase of suspension viscosity is not related to the increase of solid loading. Copper II nitrate is characterized by three orders of magnitude higher solubility in water in comparison with copper II acetylacetonate. The amount of copper nitrate which was added to the suspension was completely dissolved in water. Thus, there was no increase in solid loading in the suspension. However, the hydrolysis of copper nitrate in water leads to the increase of the concentration of Cu2+ cations and NO −3 anions. Higher concentration of ions leads to the increase of ionic strength in a suspension. The increase of salt concentration compresses the double layer around ceramic particles and, as a results, reduces repulsion forces of double layer [35]. In other words, the increase of the salt concentration decreases the height of the primary maximum of potential curve (DLVO theory). This maximum of total energy interaction provides the mechanism of stability of colloidal particles and creates an effective activation energy of aggregation [36]. Thus, when the maximum is lower, the aggregation of powder in the suspension occurs more easily which leads to the increase of the viscosity of the suspension. Above certain salt concentration, the maximum of potential curve exhibits values below zero (no energy barrier). Thus, all collisions of particles in suspension lead to aggregation. However, not only the solubility in water or concentration of salts influence the stability and the viscosity of the suspensions. The attempt to obtain 0.1 mass % of Cu by the addition of copper II acetate was also unsuccessful. The suspension was characterized by very high viscosity. Copper II acetate is a salt of high solubility in water (7.2 g·100 mL−1) which is one order of magnitude higher than copper acetylacetonate and almost two order of magnitude lower than copper II nitrate. However, values of solubility of copper II acetate were high enough to dissolve the quantity which was added to the suspension. Thus, like in case of the addition of copper II nitrate, there is no increase in solid loading in the slurry. The hydrolysis of copper II acetate in water leads to the formation of Cu2+ cations and CH3COO− anions. Cations in both salts (acetate and nitrate) are of course the same. Thus, the differences in rheological properties of ZnO-based suspensions containing copper nitrate and acetate is related to the anion group. The CH3COO− and NO −3 differ in size, shape as well as type of atoms. It seems that CH3COO− anions disturb the stability of ZnO-based aqueous suspensions more efficiently in comparison with NO −3 anions.
The performed investigations revealed that copper II nitrate, as salt well soluble in water, can be efficient additive when small amounts of dopant and its homogeneous distribution are required. However, when the concentation of a dopant should be higher, the use of non-soluble acetylacetonate is more suitable. Moreover, salts of high solubility in water cannot be used in slip casting, since in this method the solvent is filtrated from the slurry through a permeable mould by capillary forces [15]. Thus, cations and ions of hydrolysed salt will be also removed, together with water. The use of water-soluble salts as dopant ‘carriers’ should take place in methods where water is removed by evaporation: gelcasting, direct coagulation casting or tape casting methods. The acetylacetonates are more universal ‘carriers’ of dopants and can be used also in slip casting, since particles of this salt will not be removed by porous mould. Because of this fact, as well as because of the possibility to obtain higher concentration of the dopant, ZnO-based suspensions with the addition of copper II acetylacetonate have been used to shape green samples by slip casting method.
Density of green and sintered samples
The density of green bodies of pure ZnO and ZnO with copper II acetylacetonate (0.1 mass % of Cu) were very high and equalled to 59.7% of TD and 57.4% of TD, respectively (Table 2). The addition of 2 mass % of Cu in the form of this salt results in slight decrease of density to 53.7% of TD which is still high value. The density of sample in a green state is the important parameter since it influences on drying and sintering processes [37]. Higher density of samples results in lower values of shrinkage during drying as well as shorter drying time. The green samples were subjected to two-step sintering process at temperatures 1200/1150 °C for 90 min, with heating rate of 3 °C·min−1.
The densities of sintered samples are shown in Table 2. The highest density, 98% of TD was obtained for pure ZnO sample. This value is high, thus sintering parameters were chosen properly and allowed to obtain sample of almost no porosity. The addition of Cu in the form of acetylacetonates slightly decreases the density of the sintered samples. However, this effect was almost negligible when the addition of the salt was small (0.1 mass % of Cu). For the higher concentration of Cu (2 mass %), significant decrease in density was observed (83.8% of TD). Table 2 shows also the values of linear and volumetric shrinkage of ZnO samples. The lowest shrinkage was observed for ZnO sample without the dopant. The addition of copper II acetylacetonate increases the shrinkage during sintering. This phenomenon could be explained by differences in densities of green samples. Lower values of densities in a green state results in the higher shrinkage during sintering [38]. However, it is interesting that despite the fact of the highest shrinkage, the sample with 2 mass % of Cu exhibits much lower density after sintering in comparison with other samples. The influence of copper II acetylacetonate on sinterability of ZnO powder is related to the two factors. The first one is the presence of Cu atoms/ions in crystal structure of ZnO or at boundaries of ZnO grains. The second one is the role of gases which are formed during decomposition of the copper salt during thermal treatment. In order to understand the role of each factor, the microstructure observations have been performed by using light and scanning electron microscopy.
Microstructure observations
Figure 8 shows the microstructure of the sintered samples. The surface of the samples was mechanically polished followed by thermal etching (50 °C below the sintering temperature). The ZnO sample without the addition of Cu was characterized by homogeneous microstructure composed of small, polyhedral grains (Fig. 8a). Only small pores in triple-points between grains were observed. The average grain size was 1.85 µm. The addition of 0.1 mass % of Cu increases significantly the grain size to the value of 4.75 µm (Fig. 8b) which is 2.5 times higher than for the sample without the dopant. The increase of the concentration of Cu to 2 mass % induced subsequent grain growth (Fig. 8c). The grain size of the sample with the highest Cu concentration was 6.72 µm. Thus, the difference in grain size was smaller between samples with 0.1 mass % and 2 mass % of Cu in comparison with the difference between sample without Cu and with the smallest concentration of Cu. Interesting is fact that for all microstructures presented in Fig. 8 the morphology of grains, except for the grain size, is similar. This is also indicated by similar values of circularity, elongation and shape factors for all samples. On the other hand, the addition of Cu changes the grain size distribution (Fig. 9). The microstructure of the ZnO samples with Cu is characterized by wider grain size distribution in comparison with ZnO without the dopant. Moreover, the modal value (the most frequent value of a random variable) of size distribution was shifted towards higher values of grain size while its intensity (number of grains within modal value) became smaller. However, in case of sample with 2 mass % Cu bi-modal distribution occurs. The first modal value is in the range of 4.8–5.6 µm, while the second one is between grain size of 7.2–7.8 µm.
The performed observations as well as quantitative analysis of the microstructure revealed that the addition of Cu atoms facilitates the grain growth in ZnO samples. Since the morphology of the grains was similar, this effect cannot be attributed to the change of growth mechanism, but rather to the increase of diffusion rate. These results also suggest that copper was dissolved in ZnO crystal structure. Otherwise, if the copper was segregated at the grain boundaries in the form of oxide, grain boundary mobility would decrease and the grain growth would be limited. Moreover, the SEM observations did not evidence any additional phase at the grain boundaries of ZnO (Fig. 10). On the other hand, higher diffusion, grain boundary mobility and grain growth in Cu-doped ZnO sample did not lead to high density, especially in case of higher concentration of Cu (2 mass %). Higher porosity was confirmed by both density measurements (Table 2) and microstructure observations (Fig. 8b, c). The sample with the addition of 0.1 mass % of Cu was characterized by high value of density, similarly to the values of pure ZnO. However, microstructure observation (Fig. 8b) revealed the presence of dark areas which are assigned to pores. The smaller ones, located in triple-points between grains, originate from sintering process. The bigger “pores”, which size is comparable to the grain size, appeared during preparation of the surface for microscopic observations (mechanical gridding and polishing). During this process, some grains were pulled out from the surface. This was confirmed by SEM observations, where pores of sharp edges with size and shape of grains were observed (Fig. 10a). Figure 10 presents also the pores located in triple-points of the grain in pure ZnO sample as well as with the addition of Cu.
Nevertheless, higher porosity of ZnO sample with 2 mass % of Cu was confirmed by density measurements and microstructure observations. Despite high diffusion rate and higher grain mobility, the sintering process, in this particular case, did not lead to obtain sample of high density. The explanation of this phenomenon is probably related to the copper (II) acetylacetonate structure. This salt, beside copper, is composed of acetylacetone anions which decomposed to H2O, CO2 and other compounds at elevated temperatures (Fig. 2, 5). The molecular weight of copper is 63,546 u, which is about 24% of molecular weight of copper II acetylacetonate. Thus, in order to obtain concentration of 2 mass % of Cu, 8.3% by mass of C10H16CuO4 should be added to the ZnO suspension. This means, that 6 mass % of this dopant is decomposed during sintering. Taking into consideration low density of this salt, the volume percentage of evaporated/burned out material is even higher. Thus, decomposition of the copper (II) acetylacetonate leads not only to incorporation of Cu into the structure but also to formation of voids (pores) due to burn out of organic part of salt. However, according to our results obtain by thermogravimetry coupled with mass spectrometry (Fig. 2, 5) the decomposition of organic part of copper (II) acetylacetonate was observed slightly below 300 °C. Such low temperature of decomposition is beneficial in terms of sintering process, since all gases will be removed from the sample before the first stage of sintering [38]. The decomposition at sintering stage is unfavourable, since formed gases can be trapped inside the sample, caused defects or stresses or inhibit full densification [39]. However, even though the decomposition temperature of copper II acetylacetonate is sufficiently low and safe for sintering process, high porosity was observed in ZnO sample with addition of 2 mass % of Cu (8.3 mass % of C10H16CuO4). Thus, the volume of free species (pores), formed during thermal treatment, is too high to be removed during sintering. This can be related to the high concentration of the dopant or to insufficient comminution and distribution of the salt.
Conclusions
Several conclusions can be drown from this study. All investigated salts decomposed below 300 °C which is beneficial for the sintering process. In air atmosphere, the decomposition process mostly leads to simple gaseous chemical compounds, like CO2, H2O as well as NOx in case of nitrate. In argon atmosphere, the presence of more complex compounds (acetone, acetyl anions) was observed. The decomposition of salt leads also to solid products, which are Cu, Cu2O and CuO. However, the performed investigations evidenced that the decomposition of copper II nitrate leads directly to formation of CuO while in case of other salts, the copper and copper I oxide are formed followed by their oxidation to copper II oxide. In case of copper (II) acetylacetonate and acetate the thermal decomposition occurred in one step, besides the dehydration process, while in case of copper (II) nitrate the decomposition proceeds in two steps with formation of nitrogen oxide in both steps. The presence of ZnO powder resulted in change of the decomposition of copper II acetylacetonate from one to two steps in air atmosphere.
The addition of copper salts to ZnO-based suspensions had significant influence on their rheological properties. The use of salts of high solubility in water (acetate and nitrate) allowed to obtain only low concentrations of Cu (up to 0.1 mass % in case of copper (II) nitrate and below 0.1 mass % in case of copper (II) acetate) due to the increase in ionic strength and flocculation of the ZnO powder. Contrary, with the use of acetylacetonate, higher concentration of Cu (up to 2 mass %) could be achieved without significant decrease of the viscosity. However, in case of the higher concentration of dopant low densities of green (53.7% of TD) and sintered (83.8% of TD) bodies were observed. The addition of Cu increases the grain size of ZnO from 1.85 µm (pure ZnO) to 6.72 µm (ZnO with addition of 2 mass % of Cu) due to the facilitation of diffusion process and grain boundary migration. Thus, this dopant can be used as grain size modifier in ZnO-based materials.
The performed investigations revealed that metal salts can be used as dopants for ZnO process by wet forming methods. However, the form of the salt should be determined by assumed concentration. A special attention should be also paid to comminution/milling process of the salts of low solubility in water in order to obtain high distribution homogeneity.
References
Bajpai G, Moirangthem I, Sarkar S, Barman SR, Vinod CP, Bajpai S, et al. Role of Li+ and Fe3+ in modified ZnO: structural, vibrational, opto-electronic, mechanical and magnetic properties. Ceram Int. 2019;45:7232–43. https://doi.org/10.1016/j.ceramint.2019.01.004.
Qiu W, Hng HH. Effects of dopants on the microstructure and properties of PZT ceramics. Mater Chem Phys. 2002;75:151–6. https://doi.org/10.1016/S0254-0584(02)00045-7.
Wojteczko A, Lach R, Wojteczko K, Pȩdzich Z. Investigations of the subcritical crack growth phenomenon and the estimation of lifetime of alumina and alumina-zirconia composites with different phase arrangements. Ceram Int. 2016;42:9438–42. https://doi.org/10.1016/j.ceramint.2016.02.178.
Jacob AA, Balakrishnan L, Meher SR, Shambavi K, Alex ZC. Structural, optical and photodetection characteristics of Cd alloyed ZnO thin film by spin coating. J Alloys Compd. 2017;695:3753–9. https://doi.org/10.1016/j.jallcom.2016.11.265.
Meng P, Hu J, Zhao H, He J. High voltage gradient and low residual-voltage ZnO varistor ceramics tailored by doping with In2O3 and Al2O3. Ceram Int. 2016;42:19437–40. https://doi.org/10.1016/j.ceramint.2016.09.032.
Bai H, Li S, Zhao Y, Xu Z, Chu R, Hao J, et al. Influence of Cr2O3 on highly nonlinear properties and low leakage current of ZnO-Bi2O3 varistor ceramics. Ceram Int. 2016;42:10547–50. https://doi.org/10.1016/j.ceramint.2016.03.042.
Kedzierska-Sar A, Starzonek S, Kukielski M, Falkowski P, Rzoska SJ, Szafran M. Gelcasting of Al2O3–W composites: broadband dielectric spectroscopy and rheological studies of tungsten influence on polymerisation kinetics. Ceram Int. 2019;45:15237–43. https://doi.org/10.1016/j.ceramint.2019.05.012.
Znaidi L. Sol-gel-deposited ZnO thin films: a review. Mater Sci Eng B Solid-State Mater Adv Technol. 2010;174:18–30. https://doi.org/10.1016/j.mseb.2010.07.001.
Park S, Kim S, Kheel H, Hyun SK, Jin C, Lee C. Enhanced H2S gas sensing performance of networked CuO–ZnO composite nanoparticle sensor. Mater Res Bull. 2016;82:130–5. https://doi.org/10.1016/j.materresbull.2016.02.011.
Scarlat O, Mihaiu S, Aldica G, Zaharescu M, Groza JR. Enhanced properties of tin (IV) oxide based materials by field-acticated sintering. J Am Ceram Soc. 2003;86:893–7. https://doi.org/10.1111/j.1151-2916.2003.tb03393.x.
Rubenis K, Populoh S, Thiel P, Yoon S, Müller U, Locs J. Thermoelectric properties of dense Sb-doped SnO2 ceramics. J Alloys Compd. 2017;692:515–21. https://doi.org/10.1016/j.jallcom.2016.09.062.
Özgür Ü, Alivov YI, Liu C, Teke A, Reshchikov MA, Doǧan S, et al. A comprehensive review of ZnO materials and devices. J Appl Phys. 2005;98:1–103. https://doi.org/10.1063/1.1992666.
Wang X, Zhao M, Liu F, Jia J, Li X, Cao L. C2H2 gas sensor based on Ni-doped ZnO electrospun nanofibers. Ceram Int. 2013;39:2883–7. https://doi.org/10.1016/j.ceramint.2012.09.062.
Izoulet A, Guillemet-Fritsch S, Estournès C, Morel J. Microstructure control to reduce leakage current of medium and high voltage ceramic varistors based on doped ZnO. J Eur Ceram Soc. 2014;34:3707–14. https://doi.org/10.1016/j.jeurceramsoc.2014.05.033.
Moreno R. Better ceramics through colloid chemistry. J Eur Ceram Soc. 2020;40:559–87. https://doi.org/10.1016/j.jeurceramsoc.2019.10.014.
Evans JRG. Seventy ways to make ceramics. J Eur Ceram Soc. 2008;28:1421–32. https://doi.org/10.1016/j.jeurceramsoc.2007.12.015.
Vaka M, Walvekar R, Khalid M, et al. Low-melting-temperature binary molten nitrate salt mixtures for solar energy storage. J Therm Anal Calorim. 2020;141:2657–64. https://doi.org/10.1007/s10973-020-09683-y.
Vaka M, Walvekar R, Khalid M, et al. Synthesis of hybrid graphene/TiO2 nanoparticles based high-temperature quinary salt mixture for energy storage application. J Energy Storage. 2020;31:101540. https://doi.org/10.1016/j.est.2020.101540.
Faisal AS, Khalid M, Walvekar R, Vaka M, et al. Investigating the effect of graphene on eutectic salt properties for thermal energy storage. Mater Res Bull. 2019;119:110568. https://doi.org/10.1016/j.materresbull.2019.110568.
Vitali S, Giorgini L. Overview of the rheological behaviour of ceramic slurries. FME Trans. 2019;47:42–7. https://doi.org/10.5937/fmet1901042V.
Pietrzak E, Wieclaw-Midor A, Wiecinska P, Poterala M, Szafran M. Thermal decomposition of polyhydroxy processing agents dedicated to colloidal shaping of ceramics: thermogravimetry coupled with mass spectrometry and properties of ZTA composites. Thermochim Acta. 2019;674:100–9. https://doi.org/10.1016/j.tca.2019.02.011.
Wacławska I, Szumera M, Sułowska J. Thermal and structural interactions in transition elements containing silicate-phosphate glasses. Thermochim Acta. 2014;593:71–5. https://doi.org/10.1016/j.tca.2014.08.028.
Kasprzyk M, Środa M, Szumera M. Influence of Gd2O3 on thermal stability of oxyfluoride glasses. J Therm Anal Calorim. 2017;130:207–20. https://doi.org/10.1007/s10973-017-6354-9.
Lalancette RA, Syzdek D, Grebowicz J, Arslan E, Bernal I. The thermal decomposition and analyses of metal tris-acetylacetonates: free radical formation from Al, Cr, Mn, Fe and Co complexes. J Therm Anal Calorim. 2019;135:3463–70. https://doi.org/10.1007/s10973-018-7598-8.
Lin Z, Han D, Li S. Study on thermal decomposition of copper(II) acetate monohydrate in air. J Therm Anal Calorim. 2012;107:471–5. https://doi.org/10.1007/s10973-011-1454-4.
Shiota K, Matsunaga H, Miyake A. Thermal analysis of ammonium nitrate and basic copper(II) nitrate mixtures. J Therm Anal Calorim. 2015;121:281–6. https://doi.org/10.1007/s10973-015-4536-x.
Yoda S, Takebayashi Y, Sue K, Furuya T, Otake K. Thermal decomposition of copper (II) acetylacetonate in supercritical carbon dioxide: in situ observation via UV–vis spectroscopy. J Supercrit Fluids. 2017;123:82–91. https://doi.org/10.1016/j.supflu.2016.12.017.
Turgambaeva AE, Bykov AF, Igumenov IK. Investigation of the thermal decomposition of bis(acetylacetonato)copper(II) vapour by a mass spectrometric method. Thermochim Acta. 1995;256:443–56. https://doi.org/10.1016/0040-6031(95)91302-Y.
Nasibulin AG, Richard O, Kauppinen EI, Brown DP, Jokiniemi JK, Altman IS. Nanoparticle synthesis by copper (II) acetylacetonate vapor decomposition in the presence of oxygen. Aerosol Sci Technol. 2002;36:899–911. https://doi.org/10.1080/02786820290038546.
Yi F, DeLisio JB, Nguyen N, Zachariah MR, LaVan DA. High heating rate decomposition dynamics of copper oxide by nanocalorimetry-coupled time-of-flight mass spectrometry. Chem Phys Lett. 2017;689:26–9. https://doi.org/10.1016/j.cplett.2017.09.066.
Bellini JV, Machado R, Morelli MR, Kiminami RHGA. Thermal, structural and morphological characterisation of freeze-dried copper (II) acetate monohydrate and its solid decomposition products. Mater Res. 2002;5:453–7. https://doi.org/10.1590/S1516-14392002000400010.
Ryu S, Lee W, Park S. Thermal decomposition of hydrated copper nitrate on activated carbon fibers. Carbon Sci. 2004;5:180–5.
Yamada H, Soejima Y, Zheng XG, Kawaminami M. Trans Mater Res Soc Jpn. 2000;25:1199–202.
Wiecinska P, Wieclaw A, Bilski F. Selected sugar acids as highly effective deflocculants for concentrated nanoalumina suspensions. J Eur Ceram Soc. 2017;37:4033–41. https://doi.org/10.1016/j.jeurceramsoc.2017.05.037.
Verwey EJW, Overbeek JTG, van Ness K. Theory of the stability of lyophobic colloids. The interaction of particles having an electric double layer. Amsterdam: Elsevier; 1949.
Cosgrove T. Colloid Science: Principles, Methods and Applications. 2nd ed. Blackwell: Wiley; 2010.
Occhionero MA, Halloran JW. (1984) The influence of green density upon sintering. In: Kuczynski GC, Miller AE, Sargent GA, editors. Materials Science Research. Springer: New York; 1984. p. 89–102.
Norris LF, Parravano G. Sintering of zinc oxide. J Am Ceram Soc. 1963;46:449–52.
Wang K, Qiu M, Jiao C, Gu J, Xie D, Wang C, et al. Study on defect-free debinding green body of ceramic formed by DLP technology. Ceram Int. 2020;46:2438–46. https://doi.org/10.1016/j.ceramint.2019.09.237.
Acknowledgements
This work was supported by the National Science Centre, Poland [Grant No. 2016/23/D/ST8/02687]
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wiecinski, P., Wieclaw-Midor, A. Metal salts as dopants for ZnO ceramics-thermogravimetry coupled with mass spectrometry studies. J Therm Anal Calorim 147, 5599–5615 (2022). https://doi.org/10.1007/s10973-021-10934-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10934-9