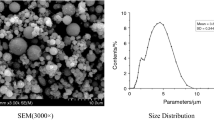
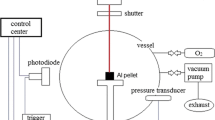
Abstract
The processes of ignition and combustion of aluminum (Al) under different pressure conditions have received widespread attention. In the present work, experiments were performed in a mixed O2/CO2 atmosphere and at five different pressures (1 atm, 5 atm, 9 atm, 13 atm and 17 atm) to study the ignition and combustion of aluminum. The microstructure and burning rate of the condensed phase products were determined using scanning electron microscopy and inductively coupled plasma spectrometer. The results showed that, with the increase in pressure, the ignition delay time of the sample shortened, while the combustion temperature, heat release rate, maximum intensity of the emission spectrum and burnout rate gradually increased. Experiments showed that at the pressure of 17 atm, the minimum ignition delay time (36 ms) achieved. At the same time, the combustion temperature and maximum burnout rate arrived at their maximum, which are 1855 °C and 99.53%, respectively. However, under high- and low-pressure conditions, there were two distinct reaction mechanisms. One was the melt-dispersion reaction (rupture of the oxide layer), which occurred under high pressure, while the other was the diffusion reaction (molecular diffusion), which took place under low pressure. In addition, physical models of the Al sample under high- and low-pressure conditions were established. The ignition temperature of Al in O2/CO2 atmosphere at 1 atm was about 930 °C. The spatial distributions of Al and AlO radicals under different pressures were found to be similar. The radicals were more concentrated on the surface of the sample, while free radicals diffused into the gas phase and reacted only under high temperature.











Similar content being viewed by others
References
Mohan S, Furet L, Dreizin EL. Aluminum particle ignition in different oxidizing environments. Combust Flame. 2010;157(7):1356–63.
Badiola C, Gill RJ, Dreizin EL. Combustion characteristics of micron-sized aluminum particles in oxygenated environments. Combust Flame. 2011;158(10):2064–70.
Gill RJ, Badiola C, Dreizin EL. Combustion times and emission profiles of micron-sized aluminum particles burning in different environments. Combust Flame. 2010;157(11):2015–23.
Zhou Y, Liu J, Wang J, Xv T, Wang J, Zhou J, Cen K. Experimental study on dynamic combustion characteristics of aluminum particles. Propell Explos Pyrot. 2017;42(8):982–92.
Bazyn T, Krier H, Glumac N. Oxidizer and pressure effects on the combustion of 10-micron aluminum particles. J Propul Power. 2005;21(4):577–82.
Bazyn T, Krier H, Glumac N. Combustion of nanoaluminum at elevated pressure and temperature behind reflected shock waves. Combust Flame. 2006;145(4):703–13.
Wilson RP Jr, Williams FA. Experimental study of the combustion of single aluminum particles in O2/Ar. Symp Combust. 1971;13(1):833–45.
Olsen SE, Beckstead MW. Burn time measurements of single aluminum particles in steam and CO2 mixtures. J Propul Power. 2011;12(4):662–71.
Roberts TA, Burton RL, Krier H. Ignition and combustion of aluminummagnesium alloy particles in O2 at high pressures. Combust Flame. 1993;92(93):125–43.
Trunov MA, Schoenitz M, Zhu X, Dreizin EL. Effect of polymorphic phase transformations in Al2O3 film on oxidation kinetics of aluminum powders. Combust Flame. 2005;140(4):310–8.
Brooks KP, Beckstead MW. Dynamics of aluminum combustion. J Propul Power. 1995;11(4):769–80.
King MK. Modeling of single particle aluminum combustion in CO2−N2 atmospheres. Symp Int Combust. 1979;17(1):1317–28.
Law CK. A simplified theoretical model for the vapor phase combustion of metal particles. Combust Sci Technol. 1973;7(5):197–212.
Benkiewicz K, Hayashi AK. Aluminum dust ignition behind reflected shock wave: two-dimensional simulations. Fluid Dyn Res. 2002;30(5):269–92.
Boiko VM, Poplavski SV. Self-ignition and ignition of aluminum powders in shock waves. Shock Waves. 2002;11(4):289–95.
Sundaram DS, Puri P, Yang V. A general theory of ignition and combustion of nano- and micron-sized aluminum particles. Combust Flame. 2016;169:94–109.
Glassman I. Combustion. 3rd ed. San Diego: Acadamic Press; 1996.
Bucher P, Yetter RA, Dryer FL, Vicenzi EP, Parr TP, Hanson-Parr DM. Condensed-phase species distributions about Al particles reacting in various oxidizers. Combust Flame. 1999;117(1–2):351–61.
Maček A. Fundamentals of combustion of single aluminum and beryllium particles. Symp Combust. 1966;11(1):203–17.
Rossi S, Dreizin EL, Law CK. Combustion of aluminum particles in carbon dioxide. Combust Sci Technol. 2001;164(1):209–37.
Driscoll J, Nicholls J, Patel V, Khatibshahidi B. Shock tube study of the ignition and combustion of aluminum. In: Joint propulsion conference, 20th. Cincinnati, OH; 1984. p. 63–72.
Schlöffel G, Eichhorn A, Albers H, Mundt C, Seiler F, Zhang F. The effect of a shock wave on the ignition behavior of aluminum particles in a shock tube. Combust Flame. 2010;157(3):446–54.
Chintersingh KL, Schoenitz M, Dreizin EL. Oxidation kinetics and combustion of boron particles with modified surface. Combust Flame. 2016;173:288–95.
Zhou Y, Liu J, Liang D, Shi W, Yang W, Zhou J. Effect of particle size and oxygen content on ignition and combustion of aluminum particles. Chin J Aeronaut. 2017;30(6):1835–43.
Mohan S, Trunov MA, Dreizin EL. Heating and ignition of metal particles in the transition heat transfer regime. J Heat Transf. 2008;130(10):1542–7.
Zhang S, Dreizin EL. Reaction interface for heterogeneous oxidation of aluminum powders. J Phys Chem C. 2013;117(27):14025–31.
Levitas VI, Pantoya ML, Dikici B. Melt dispersion versus diffusive oxidation mechanism for aluminum nanoparticles: critical experiments and controlling parameters. Appl Phys Lett. 2008;92(1):64903.
Yamamoto K, Ozeki M, Hayashi N, Yamashita H. Burning velocity and OH concentration in premixed combustion. Proc Combust Inst. 2009;32(1):1227–35.
Acknowledgements
This work was funded by the Research Program Foundation of Nanjing Institute of Technology (YKJ 201998), the National Natural Science Foundation of China (No. 51706057) and Aerospace Science and Technology Foundation of China (No. 6141B06260642).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qiu, Q., Zhou, Y., Liu, J. et al. Combustion of aluminum powder using CO2 laser in O2/CO2 atmosphere under different pressure conditions. J Therm Anal Calorim 147, 4959–4970 (2022). https://doi.org/10.1007/s10973-021-10910-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10910-3