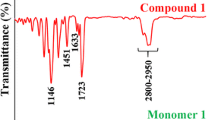
Abstract
This work investigates in depth the thermal degradation process of a polymer of urethane dimethacrylate (UDMA). UDMA monomer has been widely used in dental restorations and biomaterials. The use of density functional theory (DFT) calculations provided the bases for understanding the structure and reactivity of the UDMA monomer. Simultaneous thermogravimetry–differential thermal analysis, Photovisual Differential Scanning Calorimetry, and mid-infrared spectroscopy (MIR) were used to examine the depolymerization and degradation process. Non-isothermal kinetics made it possible to determine the best fit (n-dimensional nucleation according to Avrami–Erofeev followed by two competitive processes: nth order with autocatalysis by-product and reaction of nth order). Furthermore, the UDMA-P lifetime (5%) was calculated to show a degradation time of 3 years at 100.0 °C. Notwithstanding, techniques such as MIR and nuclear magnetic resonance 13C, 1H linked to DFT calculations helped to elucidate the cleavage positions and possible degradation by-products of UDMA degradation.









Similar content being viewed by others
References
Moszner N, Salz U. New developments of polymeric dental composites. Prog Polym Sci. 2001;26:535–76.
Siderou I, Tserki V, Papanastasiou G. Effect of chemical structure on degree of conversion in light cured dimethacrylate-based dental resins. Biomaterial. 2002;23:1819–29.
Moszner N, Fisher UK, Angermann J, Rheinberger V. A partially aromatic urethane dimethacrylate for Bis-GMA in restorative composites. Dent Mater. 2008;24:694–9.
Moszner N, Salz U, Zimmermann J. Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater. 2005;21:895–910.
Du M, Zheng Y. Modification of Silica nanoparticles and their application in UDMA dental polymeric composites. Polym Composite. 2007;28:198–207.
Floyd CJE, Dickens SH. Network structure of Bis-GMA and UDMA-based resin systems. Dent Mater. 2006;22:1143–9.
Ferracane JL. Resin composite-state of the art. Dent Mater. 2011;27:29–38.
Atai M, Ahmadi M, Babanzadeh S, Watts DC. Synthesis, characterization, shrinkage and curing kinetics of a new low-shrinkage urethane dimethacrylate monomer for dental applications. Dent Mater. 2007;23:1030–41.
Landuyt KLV, Snauwaert J, Munc JD, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Meerbeek BV. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–85.
Junling W, Xie X, Zhao H, Tay FR, Weir MD, Melo MAS, Oates TW, Zhang N, Zhang Q, Xu HHK. Development of a new class of self-healing and therapeutic dental resins. Polym Degrad Stabilit. 2019;163:87–99.
Alarcon RT, Santos GC, Oliveira AR, Silva-Filho LC, Bannach G. Synthesis of luminescent polymers in the UV light region from dimethacrylate monomer using novel quinoline dyes. J Appl Polym Sci. 2019;136:47461.
Gupta SK, Saxena P, Pant VA, Pant AB. Release and toxicity of dental resin composite. Toxicol Intern. 2012;19(3):225–34.
Pomes B, Derue I, Lucas A, Nguyen JF, Richaud E. Water ageing of urethane dimethacrylate networks. Polym Degrad Stabil. 2018;154:195–202.
Altintas SH, Usumez A. Evaluation of monomer leaching from a dual cured resin cement. J Biomed Mater Res B Appl Biomater. 2008;86(2):523–9.
Vervliet P, Plas JVP, Nys SD, Duca RC, Boonen I, Elskens M, Landuyt KLV, Covaci A. Investigating the in vitro metabolism of the dental resin monomers BisGMA, BisPMA, TCD-DI-HEA and UDMA using human liver microsomes and quadrupole time of flight mass spectrometry. Toxicol. 2019;420:1–10.
Maeng WY, Jeon JW, Lee JB, Lee H, Koh YH, Kim HE. Photocurable ceramic/monomer feedstocks containing terpene crystals as sublimable porogen for UV curing-assisted 3D plotting. J Euro Ceram Soc. 2020;40:3469–77.
Yue J, Zhao P, Gerasimov JY, Lagemaat M, Grothenhuis A, Rustema-Abbing M, Mei HC, Busscher HJ, Herrmann A, Ren Y. 3D-printable antimicrobial composite resins. Advan Func Mater. 2015;25:6756–67.
Bagheri A, Jin J. Photopolymerization in 3D printing. ACS Appl Polym Mater. 2019;1:593–611.
Alarcon RT, Gaglieri C, Bannach G. Dimethacrylate polymers with different glycerol content: thermal study, degree of conversion, and morphological features. J Therm Anal Calorim. 2018;132(3):1579–91.
Sbirrazzuoli N, Vincent L, Mija A, Guigo N. Integral, differential and advanced isoconversional methods complex mechanisms and isothermal predicted conversion–time curves. Chemom Intell Lab Syst. 2009;96:219–26.
Vyazovkin S, Vincent L, Sbirrazzuoli N. Thermal denaturation of collagen analyzed by isoconversional method. Macromol Biosci. 2007;7:1181–6.
Jablonskli AE, Lang AJ, Vyazovkin S. Isoconversional kinetics of degradation of polyvinylpyrrolidone used as a matrix for ammonium nitrate stabilization. Thermochim Acta. 2008;474:78–80.
Chaudary GC, Juneja HD, Gharpure MP. Thermal degradation behaviour of some metal chelate polymer compounds with bis(bidentate) ligand by TG/DTG/DTA. J Therm Anal Calorim. 2013;112:637–347.
Chaudary GC, Ali P, Gandhare NV, Tanna JA, Juneja HD. Thermal decomposition kinetics of some transition metal coordination polymers of fumaroyl bis (paramethoxyphenylcarbamide) using DTG/DTA techniques. Arab J Chem. 2019;12(7):1070–82.
Chaudary GC, Juneja HD, Gandhare NV. Evaluation of kinetic parameters from TG/DTG data of chelate polymer compounds of isophthaoyl bis (paramethoxyphenylcarbamide). J Chin Adv Mater Soc. 2013;1(4):305–16.
Moukhina E. Thermal decomposition of AIBN part C: SADT calculation of AIBN based on DSC experiments. Thermochim acta. 2015;621:25–35.
Silva JEE, Alarcon RT, Gaglieri C, Magdalena AG, Silva-Filho LC, Bannach G. New thermal study of polymerization and degradation kinetics of methylene diphenyl diisocyanate. J Therm Anal Calorim. 2018;133(3):1455–62.
Pires OAB, Alarcon RT, Gaglieri C, Silva-Filho LC, Bannach G. Synthesis and characterization of a biopolymer of glycerol and macadamia oil. J Therm Anal Calorim. 2019;137(1):161–70.
Achilias DS, Karabela MM, Siderou ID. Thermal degradation of light-cured dimethacrylate resins Part I. Isoconversional kinetic analysis Thermochim acta. 2008;472:74–83.
Achilias DS, Karabela MM, Siderou ID. Thermal degradation and isoconversional kinetic analysis of light-cured dimethacrylate copolymers. J Therm Anal Calorim. 2010;99:917–23.
Vouvoudi EC, Achilias DS, Siderou ID. Dental light-cured nanocomposites based on a dimethacrylate matrix: thermal degradation and isoconversional kinetic analysis in N2 atmosphere. Thermochim acta. 2015;599:63–72.
Stanescu PO, Florea NM, Lungu A, Iovu H. Kinetic study on the thermal degradation of UDMA-BisGMA copolymers. Mater Plast. 2011;48:148153.
Vyadzovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochin Acta. 2011;520:1–19.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:323–8.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci. 1964;6:183–95.
American Society for Testing and Materials – ASTM (1999) ASTM-E1641: Standard test method for decomposition kinetics by Thermogravimetry. American Society for Testing and Materials – ASTM, West Conshohocken.
American Society for Testing and Materials – ASTM. ASTM-E1877: Standard practice for calculating thermal endurance of materials from thermogravimetric decomposition data, 1999. American Society for Testing and Materials – ASTM, West Conshohocken.
Ditchfield RHWJ, Hehre WJ, Pople JA. Self-consistent molecular-orbital methods IX an extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys. 1971;54(2):724–8.
Borak J, Fields C, Andrews LS, Pemberton MA. Methyl methacrylate and respiratory sensitization: a critical review. Crit Rev Toxicol. 2010;41:230–68.
Gosavi SS, Gosavi SY, Alla RK. Local and systematic effects of unpolymerized monomers. Dent Res J. 2010;7:82–7.
Politzer P, Laurence PR, Jayasuriya K. Molecular electrostatic potentials: an effective tool for the elucidation of biochemical phenomena. Environ Heath Perspect. 1985;61:191–202.
Hayama T, Takahashi K, Kikutake K, Yokota I, Nemoto K. Analysis of polymerization behavior of dental dimethacrylate monomers by differential scanning calorimetry. J Oral Sci. 1999;41:9–13.
Scott G. Initiation process in polymer degradation. Polym Degrad Stabil. 1995;48:315–24.
DiNenno PJ, Drysdale D, Beyler CL, Walton WD, Custer RLP, Hall JR, Watts JM. Handbook of fire protection engineering. 3rd ed. Massachusetts: National Fire Protection Association; 2002.
Opeida A, Sheparovych RB. Inhibition by hydrogen peroxide in the radical chain oxidation of hydrocarbons by molecular oxygen. Theoric Exper Chem. 2019;55:36–42.
Decker C, Jenkins AD. Kinetic approach of O2 inhibition in ultraviolet- and laser-induced polymerization. Macromol. 1985;18:1241–4.
Bhanu VA, Kishore K. Role of oxygen polymerization reactions. Chem Rev. 1991;91:99–117.
Christmann J, Ley C, Allonas X, Ibrahim A, Croutxé-Barghorn C. Polymer. 2019;160:254–64.
Alarcon RT, Gaglieri C, da Silva BHT, da Silva LC, Bannach G. New fluorescein dye derivatives and their use as an efficient photoinitiator using blue light LED. J Photoch photobio A. 2017;343:112–8.
Alarcon RT, Gaglieri C, de Oliveira AR, Bannach G. Use of DSC in degree of conversion of dimethacrylet polymers: easier and faster than MIR technique. J Therm Anal Calorim. 2018;132:1423–7.
Ros S, Braido RS, Castro NLS, Brandão ALT, Schwaab M, Pinto JC. Modelling the chemical recycling of crosslinked poly(methyl methacrylate): Kinetics of depolymerisation. J Anal Appl Pyrol. 2019;144:104706.
Opfermann J. Kinetic analysis using a multivariate nonlinear regression. J Therm Anal Calorim. 2000;60:641–58.
Vyazovkin S, Burnham AK, Favergeon L, Koga N, Moukhina E, Pérez-Maqueda LA, Sbirrazzuoli N. ICTAC kinetics committee recommendations for analysis of multi-step kinetics. Thermochim Acta. 2020;689:178597.
Moukhina E. Determination of kinetic mechanisms for reactions measured with thermoanalytical instruments. J Therm Anal Calorim. 2012;109:1203–14.
Vyazovkin S. Kinetic effects of pressure on decomposition of solids. Inter Rev Phys Chem. 2020;39:35–66.
Helfferich FG. Kinetics of multistep reactions. 2nd ed. Amsterdam: Elsevier; 2004.
Chaudary GC, Juneja HD, Pagadala R, Gandhare NV, Gharpure MP. Synthesis, characterisation and thermal degradation behaviour of some coordination polymers by using TG–DTG and DTA techniques. J Saud Chem Soc. 2015;19:442–53.
Silverstein RM, Webster FX, Kiemle DJ. Spectrometric Identification of organic compounds. 7th ed. Rio de Janeiro: Livros Técnicos e Científicos Editora S.A; 2007.
Durán I, Ortiz P. FTIR monomer conversion analysis of UDMA-based dental resins. J Oral Rehab. 1996;23(9):632–7.
Acknowledgements
The authors wish to thank CAPES (Grants. 024/2012 Pro-equipment), POSMAT/UNESP, São Paulo State Foundation—FAPESP (Grants 2017/08820-8, 2018/03460-6, 2015/00615-0, 2016/01599-1, and 2018/14506-7), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grants. 302769/2018-8 and 301857/2018-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 830 kb)
Supplementary material 1 (MP4 2039 kb)
Rights and permissions
About this article
Cite this article
Alarcon, R.T., Gaglieri, C., dos Santos, G.C. et al. A deep investigation into the thermal degradation of urethane dimethacrylate polymer. J Therm Anal Calorim 147, 3083–3097 (2022). https://doi.org/10.1007/s10973-021-10610-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10610-y