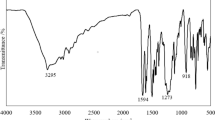
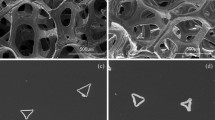
Abstract
In this paper, effects of Pt compounds (C8H18OPtSi2) on pyrolysis and oxidation characteristics of silicone foam (SiFs), gas volatiles of SiFs during pyrolysis and oxidation process, influence of heating rate on pyrolysis and oxidation of SiFs, and the thermal reaction kinetics of SiFs were analysed. The residual mass of SiFs at 900.0 °C in N2 atmosphere increased from 59.7 to 74.1% as the content of Pt compounds changed from 0.3 to 1.2 mass%. Whereas, the residual mass of SiFs in air atmosphere was higher than in N2 atmosphere. CO2 and CO as new gas volatiles appeared in air atmosphere when compared in N2 atmosphere. The residual mass of SiFs rose with the increase of heating rate. Besides, the apparent activation energy in N2 atmosphere grew significantly with conversion rate, and the apparent activation energy in air atmosphere was significantly lower than that in N2 atmosphere.














Similar content being viewed by others
References
Song LX, Lu A, Feng P, Lu ZY. Preparation of silicone rubber foam using supercritical carbon dioxide. Mater Lett. 2014;121:126–8.
Hamdani S, Longuet C, Perrin D, Lopez-cuesta J, Ganachaud F. Flame retardancy of silicone-based materials. Polym Degrad Stab. 2009;94(4):465–95.
Kang FR, Wang CP, Deng J, Yang K, Ma L, Pang QT. Flame retardancy and smoke suppression of silicone foams with microcapsulated aluminum hypophosphite and zinc borate. Polym Adv Technol. 2020;31(4):654–64.
Rabe JA, Spells S, Rasch DM, Homan GR, Lee CL. Evaluation of silicone foam for flat plate solar collector insulation. Sol Energy Mater. 1981;42(2):159–68.
Verdejo R, Saiz-Arroyo C, Carretero-Gonzalez J, BarrosoBujans F, Rodriguez-Perez A, Lopez-Manchado MA. Physical properties of silicone foams filled with carbon nanotubes and functionalized graphene sheets. Eur Polym J. 2008;44(9):2790–7.
Liu P, Liu DL, Zou HW, Fan P, Xu W. Structure and properties of closed-cell foam prepared from irradiation crosslinked silicone rubber. J Appl Polym Sci. 2009;113(6):3590–5.
Kang FR, Wang CP, Deng J, Wang WF, Li XN. Effects of talc/hollow glass beads on the flame retardancy of silicone foams. Mater Res Express. 2019;6(9):205–12.
Grande JB, Fawcett AS, McLaughlin AJ, Gonzaga F, Bender TP, Brook MA. Anhydrous formation of foamed silicone elastomers using the Pierse-Rubinsztajn reaction. Polymer. 2012;53(15):3135–42.
Deng J, Kang FR, Xiao Y, Wang WF, Shu CM, Lai WB, et al. Effects of platinum compounds/superfine aluminum hydroxide/ultrafine calcium carbonate on the flame retardation and smoke suppression of silicone foams. J Appl Polym Sci. 2020;137(1):683–93.
Chen XL, Song WK, Liu JB, Jiao CM, Qian Y. Synergistic flame-retardant effects between aluminum hypophosphite and expandable graphite in silicone rubber composites. J Therm Anal Calorim. 2015;120(3):1819–26.
Yilgör E, Yilgör I. Silicone containing copolymers: synthesis, properties and applications. Prog Polym Sci. 2014;39(6):1165–95.
Loepfe M, Schumacher CM, Stark WJ. Design performance and reinforcement of bearing-free soft silicone combustion-driven pumps. Ind Eng Chem Res. 2014;53:12519–26.
Gao BZ, Xu JZ, Peng JJ, Kang FY, Du HD, Li J, et al. Experimental and theoretical studies of effective thermal conductivity of composites made of silicone rubber and Al2O3 particles. Thermochim Acta. 2015;614:1–8.
Sim LC, Ramanan SR, Ismail H, Seetharamu KN, Goh TJ. Thermal characterization of Al2O3 and ZnO reinforced silicone rubber as thermal pads for heat dissipation purposes. Thermochim Acta. 2005;430:155–65.
Wang XL, Dou WQ. Preparation of graphite oxide (GO) and the thermal stability of silicone rubber/GO nanocomposites. Thermochim Acta. 2012;529:25–8.
Xu J, Razeeb KM, Roy S. Thermal properties of single walled carbon nanotube-silicone nanocomposites. J Polym Sci Part B. 2008;46(17):1845–52.
Deng SB, Liao W, Yang JC, Cao ZJ, Wang YZ. Flame-retardant and smoke-suppressed silicone foams with chitosan-based nanocoatings. Ind Eng Chem Res. 2016;55(27):7239–48.
Hamdani S, Longuet C, Lopez-Cuesta JM, Ganachaud F. Calcium and aluminium-based fillers as flame-retardant additives in silicone matrices: I—blend preparation and thermal properties. Polym Degrad Stab. 2010;95:1911–9.
Hamdani-Devarennes S, Pommier A, Longuet C, Lopez-Cuesta JM, Ganachaud F. Calcium and aluminium-based fillers as flame-retardant additives in silicone matrices II: analyses on composite residues from an industrial-based pyrolysis test. Polym Degrad Stab. 2011;96:1562–72.
Chen W, Zeng X, Lai X. Synergistic effect and mechanism of platinum catalyst and nitrogen-containing silane on the thermal stability of silicone rubber. Thermochim Acta. 2016;632:1–9.
Delebecq E, Hamdani-Devarennes S, Raeke J, Cuesta J, Ganachaud F. High residue contents indebted by platinum and silica synergistic action during the pyrolysis of silicone formulations. ACS Appl Mater Interfaces. 2011;3(3):869–80.
Caminoa G, Lomakinb SM, Lazzari M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer. 2001;42:2395–402.
Aacknowledgements
The work was financially supported by the National Natural Science Foundations of China (no. 5177-4232), National Key R&D Program of China (no. 2018YFC0807900), Natural Science Basic Science Research Plan of Shaanxi Province, China (2017JM-5114), Key Technology and Technology Projects for the Prevention and Control of Major Accidents in National Safety Production (Shaanxi-0005-2017AQ), and Industrial Science and Technology Research Project of Shaanxi Province, China (2016GY-191).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kang, F., Deng, J., Bai, Z. et al. Pyrolysis and oxidation behaviour of dehydrogenation silicone foam containing Pt compounds. J Therm Anal Calorim 144, 351–361 (2021). https://doi.org/10.1007/s10973-020-10360-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10360-3