Abstract
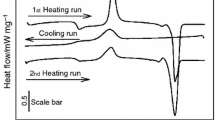
Modification of poly(lactic acid) (PLA) was performed with the surface modified magnesium hydroxide (mMH) obtained from seawater. Surface modification of MH with different amount of stearic acid (SA) results in chemically bonded SA, stearate as confirmed by Fourier transform infrared spectroscopy (FT-IR). Based on FT-IR and X-ray diffraction (XRD) analysis modified filler with a higher amount of SA was used for the composite preparation. PLA/m10MH composites were prepared using laboratory mixing extruder. Differential scanning calorimetry (DSC) was applied to study thermal properties and crystallinity of PLA/mMH composites, while the thermal stability was performed by thermogravimetric analysis (TG). According to DSC analysis, PLA crystallization is primary influenced by the filler. PLA/m10MH composites degrade in four degradation stages. With an increase of m10MH content in the composites, their thermal degradation becomes more complex and their thermal stability is getting worse. XRD and X-ray microcomputed tomography (XμCT), used to obtain structural and microstructural information about PLA/m10MH composites, revealed that addition of m10MH decreases the crystallinity of PLA, increases the porosity of the composite and produces agglomeration of mMH.
















Similar content being viewed by others
References
Auras R, Lim L-T, Selke SEM, Tsuji H, editors. Poly(lactic acid): synthesis, structures, properties, processing, and applications. Hoboken: Wiley; 2010.
Van den Oever M, Molenveld K, Van der Zee M, Bos H. Bio-based and biodegradable plastics: facts and figures: focus on food packaging in the Netherlands. Wageningen Food & Biobased Research number; 2017.
Vink ETH, Rábago KR, Glassner DA, Gruber PR. Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym Degrad Stabil. 2003;80:403–19.
Běhálek L, et al. Thermal properties and non-isothermal crystallization kinetics of biocomposites based on poly(lactic acid), rice husks and cellulose fibres. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09894-3.
Bubeck RA, Merrington A, Dumitrascu A, Smith PB. Thermal analyses of poly(lactic acid) PLA and micro-ground paper blends. J Therm Anal Calorim. 2018;131:309–16.
Li Y, Han C, Yu Y, Xiao L, Shao Y. Isothermal and non-isothermal cold crystallization kinetics of poly(L-lactide)/functionalized eggshell powder composites. J Therm Anal Calorim. 2018;131:2213–23.
Kosowska K, Szatkowski P. Influence of ZnO, SiO2 and TiO2 on the aging process of PLA fibers produced by electrospinning method. J Therm Anal Calorim. 2020;140:1769–78.
Xin S, et al. Confinement crystallization of poly(L-lactide) induced by multiwalled carbon nanotubes and graphene nanosheets. J Therm Anal Calorim. 2015;122:379–91.
Pilarska AA, Klapiszewski Ł, Jesionowski T. Recent developments in the synthesis, modification and application of Mg(OH)2 and MgO: a review. Powder Technol. 2017;319:373–407.
Kong Q, Qian H. Low-temperature synthesis of Mg(OH)2 nanoparticles from MgO as halogen-free flame retardant for polypropylene. Fire Mater. 2014;38:145–54.
Wang W, Xueliang X, Chen J. The role of acetic acid in magnesium oxide preparation via chemical precipitation. J Am Ceram Soc. 2008;91:1697–9.
Wahab R, Ansari SG, Dar MA, Kim YS, Shin HS. Synthesis of magnesium oxide nanoparticles by sol-gel process. Mater Sci Forum. 2007;558–559:983–6.
Hai-Yan Q, Min D, Shao-ming Z, Ling-Ling X. Synthesis of superfine Mg(OH)2 particles by magnesite. Mater Sci Eng A-Struct. 2007;445–446:600–3.
Sirota V, et al. Preparation of crystalline Mg(OH)2 nanopowder from serpentinite mineral. Int J Min Sci Technol. 2018;28:499–503.
Shand MA. The chemistry and technology of magnesia. Hoboken: Wiley; 2006.
Song X, Tong K, Sun S, Sun Z, Yu J. Preparation and crystallization kinetics of micron-sized Mg(OH)2 in a mixed suspension mixed product removal crystallizer. Front Chem Sci Eng. 2013;7:130–8.
Cabrera-Álvarez EN, et al. Study of the silane modification of magnesium hydroxide and their effects on the flame retardant and tensile properties of high density polyethylene nanocomposites. Polym Compos. 2014;35:1060–9.
Lih E, et al. Modified magnesium hydroxide nanoparticles inhibit the inflammatory response to biodegradable poly(lactide-co-glycolide) implants. ACS Nano. 2018;12:6917–25.
Liu L, et al. Surface modification of magnesium hydroxide and its application in flame-retardant oil-extended styrene–ethylene–butadiene–styrene/polypropylene composites. J App Polym Sci. 2019;136(47129):1–11.
Zhao P, Liu Z, Gehde M, Kuehnert I, Leuteritz A. Effect of phosphorus-containing modified magnesium hydroxide on the mechanical properties and flammability of PLA/MH composites. AIP Conf Proc. 2019;2055(050004):1–4.
Quispe-Dominguez R, Naseem S, Leuteritz A, Kuehnert I. Synthesis and characterization of MgAl-DBS LDH/PLA composite by sonication-assisted masterbatch (SAM) melt mixing method. RSC Adv. 2019;9:658–67.
Kang EY, Lih E, Kim IH, Joung YK, Han DK. Effects of poly(L-lactide-ε-caprolactone) and magnesium hydroxide additives on physico-mechanical properties and degradation of poly(L-lactic acid). Biomater Res. 2016;20(7):1–9.
Mobedi H, Nekoomanesh M, Orafaei H, Mivehchi H. Studying the degradation of poly(L-lactide) in presence of magnesium hydroxide. Iran Polym J. 2006;15:31–9.
Kum, et al. Effect of magnesium hydroxide nanoparticles with rod and plate shape on mechanical and biological properties of Poly(L-lactide) composites. Macromol Res. 2014;22:1032–41.
Kum CH, et al. Biodegradable poly(L-lactide) composites by oligolactide-grafted magnesium hydroxide for mechanical reinforcement and reduced inflammation. J Mater Chem B. 2013;1:2764–72.
Jakić J, Štefanić G, Labor M, Martinac V. Microstructural characteristics of low-temperature (1400 °C) Sintered MgO obtained from seawater. Sci Sinter. 2017;49:61–71.
Ke T, Sun X. Effects of moisture content and heat treatment on the physical properties of starch and poly(lactic acid) blends. J Appl Polym Sci. 2001;81:3069–82.
Aguirre JM, Gutiérrez A, Giraldo O. Simple route for the synthesis of copper hydroxy salts. J Braz Chem Soc. 2011;22:546–51.
Huang H, et al. Stearic acid surface modifying Mg(OH)2: mechanism and its effect on properties of ethylene vinyl acetate/Mg(OH)2 composites. J Appl Polym Sci. 2008;107:3325–31.
Focke WW, Molefe D, Labuschagne FJ, Ramjee S. The influence of stearic acid coating on the properties of magnesium hydroxide, hydromagnesite, and hydrotalcite powders. J. Mater. Sci. 2009;44:6100–9.
Gilbert M, Sutherland I, Guest A. Characterization of coated particulate fillers. J Mater Sci. 2000;35:391–7.
Liauw CM, Rothon RN, Lees GC, Iqbal Z. Flow micro-calorimetry and FTIR studies on the adsorption of saturated and unsaturated carboxylic acids onto metal hydroxide flame-retardant fillers. J Adhes Sci Technol. 2001;15:889–912.
Zhang F, Zhang H, Su Z. Surface treatment of magnesium hydroxide to improve its dispersion in organic phase by the ultrasonic technique. Appl Surf Sci. 2007;253:7393–7.
Bracconi P, Andrès C, N’diaye A, Pourcelot Y. Thermal analyses of commercial magnesium stearate pseudopolymorphs. Thermochim Acta. 2005;429:43–51.
Wesolowski M, Rojek B. Thermogravimetric detection of incompatibilities between atenolol and excipients using multivariate techniques. J Therm Anal Calorim. 2013;113:169–77.
Perinović Jozić S, Stoilova A, Jakić J, Andričić B. Preparation and thermal analysis of polylactic acid/magnesium hydroxide composites. In: 20th international conference on materials MATRIB 2019. 2019; p. 175–90.
Aoyagi Y, Yamashita K, Doi Y. Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone], and poly[(S)-lactide]. Polym Degrad Stabil. 2002;76:53–9.
McNeill IC, Leiper HA. Degradation studies of some polyesters and polycarbonates—1. Polylactide: General features of the degradation under programmed heating conditions. Polym Degrad Stabil. 1985;11:267–85.
Pielichowski K, Njuguna J. Thermal degradation of polymeric materials. Shawbury: Rapra Technology; 2005.
Zhang D, Liu Z, Li L, Zhu L. Surface modification of magnesium hydroxide with stearic acid. Adv Mater Res. 2013;602–604:1693–9.
Lerdkanchanaporn S, Dollimore D, Alexander KS. A simultaneous TG-DTA study of the degradation in nitrogen of cellulose to carbon, alone and in the presence of other pharmaceutical excipients. Thermochim Acta. 1998;324:25–32.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Perinović Jozić, S., Jozić, D., Jakić, J. et al. Preparation and characterization of PLA composites with modified magnesium hydroxide obtained from seawater. J Therm Anal Calorim 142, 1877–1889 (2020). https://doi.org/10.1007/s10973-020-10255-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10255-3