Abstract
The investigation of decomposition thermodynamics and kinetics of active electrode materials is an important tool in the development of recycling techniques for discarded lithium-ion batteries. The knowledge of thermal decomposition kinetics and thermodynamics aids the understanding and improving thermal response and can provide guidelines for the design of the thermal recycling process. Evaluation of thermal response, kinetics parameter, and thermodynamic parameters, which include activation energy, change in Gibbs free energy (ΔG), change in enthalpy (ΔH), change in entropy (ΔS) were carried out using isoconversional kinetic methods employing thermogravimetric. Comparative analysis of thermal decomposition kinetics, experimental, and indigenous carbothermal reduction in the single (LiCoO2/C) and the mixed phases cathode (LiCoO2, LiMn2O4, LiNi0.5Mn1.5O4/C) active electrode material was also carried out. The average activation energy for the thermal dissociation of mixed cathode active material is estimated as 187.6 kJ mol−1, and ΔG, ΔH, ΔS are 243 kJ mol−1, 179.9 kJ mol−1, − 0.06 kJ mol−1 K−1, respectively. Thermal dissociation and carbothermal reduction in active electrode material was investigated in 600–1000 °C and found that active electrode material decomposes and reduces above 600 °C. A simple processing comprising reduction followed by water leaching and magnetic separation was used for metallic recovery. The optimum magnetic product of mix phase cathodeactive material comprises Co: 61%, Mn: 19%, Ni: 7%, O: 13%, and lithium was recovered as Li2CO3 with Li extraction ~ 80%.
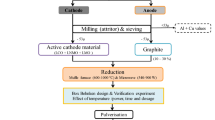
Graphic abstract








Similar content being viewed by others
Abbreviations
- LIBs:
-
Lithium-ion batteries
- LCO:
-
LiCoO2
- LMnO:
-
LiMn2O4
- LNMO:
-
LiNi0.5Mn1.5O4
- TGA:
-
Thermogravimetric Analysis
- DTG:
-
Derivative thermogravimetric analysis
- KAS:
-
Kissinger–Akahira–Sunose method
- OFW:
-
Ozawa–Flynn–Wall method
- L1:
-
Mixed electrode active material (LCO and C)
- L3:
-
Mixed electrode active material (LCO, LMnO, LNMO, and C)
References
Zeng X, Li J, Singh N. Recycling of spent lithium-ion battery: a critical review. Crit Rev Environ Sci Technol. 2014;44:1129–65.
Pinegar H, Smith YR. End-of-life lithium-ion battery component mechanical liberation and separation. J Sustain Metall. 2019;71:4447–56.
J. Mitch. It’s time to get serious about recycling lithium-ion batteries. C&EN Chem. Eng. news. 2019; 97: 1. https://cen.acs.org/materials/energy-storage/time-serious-recycling-lithium/97/i28.
Ordoñez J, Gago EJ, Girard A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew Sustain Energy Rev. 2016;60:195–205.
Sunil SR, Dhawan N. Thermal processing of spent Li-ion batteries for extraction of lithium and cobalt–manganese values. Trans Indian Inst Met. 2019;72:3035–44.
Indian Bureau of Mines, Indian Minerals Yearbook, 2018; 57. https://ibm.gov.in/index.php?c=pages&m=index&id=1373.
Winslow KM, Laux SJ, Townsend TG. A review on the growing concern and potential management strategies of waste lithium-ion batteries. Resour Conserv Recycl. 2018;129:263–77.
Meshram P, Pandey BD, Mankhand TR, Deveci H. Acid baking of spent lithium ion batteries for selective recovery of major metals: a two-step process. J Ind Eng Chem. 2016;43:117–26.
Sunil SR, Vishvakarma S, Barnwal A, Dhawan N. Processing of spent Li-ion batteries for recovery of cobalt and lithium values. JOM. 2019;71:4659–65.
Pindar S, Dhawan N. Carbothermal reduction of spent mobile phones batteries for the recovery of lithium, cobalt, and manganese values. JOM. 2019;71:4483–91.
Yun L, Linh D, Shui L, Peng X, Garg A, LE MLP, Sandoval J. Metallurgical and mechanical methods for recycling of lithium-ion battery pack for electric vehicles. Resour Conserv Recycl. 2018;136:198–208.
Nayaka GP, Pai KV, Santhosh G, Manjanna J. Recovery of cobalt as cobalt oxalate from spent lithium ion batteries by using glycine as leaching agent. J Environ Chem Eng. 2016;4(2):2378–83.
Liu P, Xiao L, Tang Y, Chen Y, Ye L, Zhu Y. Study on the reduction roasting of spent LiNixCoyMnzO2 lithium-ion battery cathode materials. J Therm Anal Calorim. 2019;136:1323–32.
Liu P, Xiao L, Chen Y, Tang Y, Wu J, Chen H. Recovering valuable metals from LiNixCoyMn1−x−yO2 cathode materials of spent lithium ion batteries via a combination of reduction roasting and stepwise leaching. J Alloys Compd. 2019;783:743–52.
Huang Z, Ruan J, Yuan Z, Qiu R. Characterization of the materials in waste power banks and the green recovery process. ACS Sustain Chem Eng. 2018;6:3815–22.
Xiao J, Li J, Xu Z. Novel approach for in situ recovery of lithium carbonate from spent lithium ion batteries using vacuum metallurgy. Environ Sci Technol. 2017;51:11960–6.
Li J, Wang G, Xu Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J Hazard Mater. 2016;302:97–104.
Hu J, Zhang J, Li H, Chen Y, Wang C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J Power Sources. 2017;351:192–9.
Xiao J, Li J, Xu Z. Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy. J Hazard Mater. 2017;338:124–31.
Vishvakarma S, Dhawan N. Recovery of cobalt and lithium values from discarded Li-ion batteries. J Sustain Metall. 2019;5:204–9.
Varma AK, Mondal P. Physicochemical characterization and pyrolysis kinetic study of sugarcane bagasse using thermogravimetric analysis. J Energy Resour Technol. 2016;138:052205.
Criado J, Sánchez-Jiménez P, Pérez-Maqueda L. Critical study of the isoconversional methods of kinetic analysis. J Therm Anal Calorim. 2008;92:199–203.
Biryan F, Pihtili G. Fabrication of a novel acrylate polymer bearing chalcone and amide groups and investigation of its thermal and isoconversional kinetic analysis. J Therm Anal Calorim. 2020;139:3857–70.
Alaba PA, Popoola SI, Abnisal F. Thermal decomposition of rice husk: a comprehensive artificial intelligence predictive model. J Therm Anal Calorim. 2020;140:1811–23. https://doi.org/10.1007/s10973-019-08915-0.
Manić N, Janković B, Dodevski V. Model-free and model-based kinetic analysis of Poplar fluff (Populus alba) pyrolysis process under dynamic conditions. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09675-y.
Singh G, Varma AK, Almas S, Jana A, Mondal P, Seay J. Pyrolysis kinetic study of waste milk packets using thermogravimetric analysis and product characterization. J Mater Cycles Waste Manag. 2019;21:1350–60.
Huang L, Liu J, He Y, Sun S, Chen J, Sun J, Kuo J. Thermodynamics and kinetics parameters of co-combustion between sewage sludge and water hyacinth in CO2/O2 atmosphere as biomass to solid biofuel. Bioresour Technol. 2016;218:631–42.
Dhaundiyal A, Singh SB. The generalisation of a multi-reaction model for polynomial ramping of temperature. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09650-7.
Xu Y, Chen B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour Technol. 2013;146:485–93.
Maia AAD, de Morais LC. Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour Technol. 2016;204:157–63.
Baruah U, Borphukan S, Saikia M, Saikia PJ, Baruah SD. Non-isothermal decomposition kinetics of in-chain functionalized poly(MMA-co-ethylene). J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09464-7.
Wang Y, Liu S, Cheng Y. Thermal analysis and hazards evaluation for HTP-65 W through calorimetric technologies and simulation. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09630-x.
Singh RK, Ruj B. Time and temperature depended fuel gas generation from pyrolysis of real world municipal plastic waste. Fuel. 2016;174:164–71.
Huang X, Cao JP, Zhao XY, Wang JX, Fan X, Zhao YP, Wei XY. Pyrolysis kinetics of soybean straw using thermogravimetric analysis. Fuel. 2016;169:93–8.
He Y, Chang C, Li P, Han X, Li H, Fang S, Ma X. Thermal decomposition and kinetics of coal and fermented cornstalk using thermogravimetric analysis. Bioresour Technol. 2018;259:294–303.
Liu W, Zhong X, Han J, Qin W, Liu T, Zhao C, Chang Z. Kinetic study and pyrolysis behaviors of spent LiFePO4 batteries. ACS Sustain Chem Eng. 2018;7:1289–99.
Ouyang D, He Y, Chen M, Liu J, Wang J. Experimental study on the thermal behaviors of lithium-ion batteries under discharge and overcharge conditions. J Therm Anal Calorim. 2018;132:65–75.
Wang Q, Ping P, Zhao X, Chu G, Sun J, Chen C. Thermal runaway caused fire and explosion of lithium ion battery. J Power Sources. 2012;208:210–24.
Bach QV, Chen WH. Pyrolysis characteristics and kinetics of microalgae via thermogravimetric analysis (TGA): a state-of-the-art review. Bioresour Technol. 2017;246:88–100.
Ye G, Luo H, Ren Z, Ahmad MS, Liu CG, Tawab A, Mehmood MA. Evaluating the bioenergy potential of Chinese liquor-industry waste through pyrolysis, thermogravimetric, kinetics and evolved gas analyses. Energy Convers Manag. 2018;163:13–21.
Kim YS, Kim YS, Kim SH. Investigation of thermodynamic parameters in the thermal decomposition of plastic waste–waste lube oil compounds. Environ Sci Technol. 2010;44:5313–7.
Acknowledgements
The authors gratefully acknowledge the funding received from the Indian Institute of Technology, Roorkee, under Faculty Initiation Grant; FIG-100714 and MHRD-UAY grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pindar, S., Dhawan, N. Kinetics and thermodynamical evaluation of electrode material of discarded lithium-ion batteries and its impact on recycling. J Therm Anal Calorim 146, 1819–1831 (2021). https://doi.org/10.1007/s10973-020-10139-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10139-6