Abstract
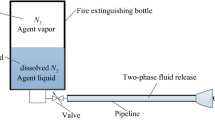
Nitrogen used for pressurization in the extinguisher can be partially dissolved in the fire extinguishing agent. Consequently, the evolution of the dissolved nitrogen has a significant effect on the release behavior of the fire extinguishing agent in a rapid process. In this article, a new model was developed to predict the critical pressure of the nitrogen evolution and the release process of the fire extinguishing agent was described in detail. According to the Peng–Robinson equation of state and van der Waals mixing rule, the effect of the dissolved nitrogen on the surface tension of the fire extinguishant was analyzed by considering surface phase and fugacity coefficient. A method to calculate the surface tension of the liquid agent dissolved with nitrogen was proposed. The results showed that the proposed model can determine the accurate critical pressure of the evolution of the dissolved nitrogen and further evaluated whether nitrogen escapes. At different initial filling pressures, in addition, the release process of the nitrogen extinguishant such as CF3I, FC218 (C3F8), HFC125 (C2HF5) and Halon1301 (CF3Br) was well predicted by the fluid release model when taking the surface tension and adiabatic index of the mixture into account. Compared with the previously obtained experimental data, the predictions obtained indicated that the present model can adequately describe the liquid and the gas mixture release stage in the release process of the nitrogen extinguishant.







Similar content being viewed by others
Abbreviations
- a :
-
Cohesive energy parameter in the PR equation of state (Pa m6 mol−2)
- A :
-
Surface area (m2); constant defined in Eq. (9)
- b :
-
Volumetric parameter in the PR equation of state (m3 mol−1)
- B :
-
Constant defined in Eq. (9)
- f :
-
Fugacity (Pa)
- J :
-
Nucleation rate (nuclei cm−3 s−1)
- k :
-
Binary interaction parameter
- M :
-
Molecular mass (g)
- N A :
-
Avogadro constant (6.02 × 1023 mol−1)
- p :
-
Pressure (Pa)
- p e :
-
Bubble-point pressure (Pa)
- R :
-
Molar gas constant (8.3145 J mol−1 K−1)
- T :
-
Absolute temperature (K)
- T i :
-
Initial temperature (K)
- v :
-
Molar volume (m3 mol−1)
- x, X :
-
Mole fraction
- y, Y :
-
Mole fraction
- Z :
-
Compressibility factor
- V :
-
Volume (m3)
- α :
-
Function of temperature in the PR equation of state
- α ij :
-
Binary parameter
- κ :
-
Function of the acentric factor
- φ :
-
Fugacity coefficient
- ω :
-
Acentric factor
- G :
-
Gas
- L :
-
Liquid
- 1:
-
Nitrogen
- 2:
-
Agent
- B:
-
Bulk phase
- c:
-
Critical point
- i, j:
-
Component identification
- m:
-
Mixture
- r:
-
Reduced parameter
- S:
-
Surface phase
- b:
-
Bottle
References
Takahashi F, Katta VR, Linteris GT, Babushok VI. A computational study of extinguishment and enhancement of propane cup-burner flames by halon and alternative agents. Fire Saf J. 2017;91:688–94.
Zhang T, Liu H, Han Z, Wang Y, Guo Z, Wang C. Experimental study on the synergistic effect of fire extinguishing by water and potassium salts. J Therm Anal Calorim. 2019;138:857–67.
Gann RG. Guidance for advanced fire suppression in aircraft. Fire Technol. 2008;44(3):263–82.
Hodgs SE, McCormick SJ. Fire extinguishing agents for protection of occupied spaces in military ground vehicles. Fire Technol. 2013;49(2):379–94.
Grosshandler WL, Gann RG, Pitts WM. Evaluation of alternative in-flight fire suppressants for full-scale testing in simulated aircraft engine nacelles and dry bays. NIST SP-861, Washington DC; 1994.
Saso Y, Saito N, Liao C, Ogawa Y. Extinction of counterfiow diffusion flames with halon replacements. Fire Saf J. 1996;26(4):303–26.
Elliott DG, Garrison PW, Klein GA, Moran KM, Zydowicz MP. Flow of nitrogen pressurized halon 1301 in fire extinguishing Systems. JPL Publication 84-62, Jet Propulsion Laboratory; 1984.
Yang JC, Cleary TG, Vázquez I, Boyer CI, King MD, Breuel BD, Gmurczyk G. Optimization of system discharge. In: Fire suppression system performance of alternative agents in aircraft engine and dry bay laboratory simulations, NIST SP-890, Washington DC, 1995. pp 407–782.
Yang JC, Cleary TG, Huber ML, Grosshandler WL. Vapour nucleation in a cryogenic—fluid-dissolved-nitrogen mixture during rapid depressurization. The Royal Society. 1999;455:1717–38.
Blander M, Katz JL. Bubble nucleation in liquids. AIChE J. 1975;21(5):833–48.
Forest TW, Ward CA. Homogeneous nucleation of bubbles in solutions at pressures above the vapor pressure of the pure liquid. J Chem Phys. 1978;69(5):2221–30.
Schmelzer WP, Baidakov G, Boltachev S. Kinetics of boiling in binary liquid-gas solutions: comparison of different approaches. J Chem Phys. 2003;119(12):6166–83.
Němec T. Homogeneous bubble nucleation in binary systems of liquid solvent and dissolved gas. Chem Phys. 2016;467:26–37.
Jiang W, Bian J, Liu Y, Gao S, Chen M, Du S. Modification of the CO2 surface tension calculation model under low-temperature and high-pressure condition. J Dispersion Sci Technol. 2017;38(5):671–6.
Duan Y, Zhang C, Lin H, Zhu M. The prediction of surface tension for HFCs and HCFCs. J Eng Thermophys. 2001;22(3):278–80 (in Chinese).
Nicola GD, Moglie M. A generalized equation for the surface tension of refrigerants. Int J Refrig. 2011;34(4):1098–108.
Nicola GD, Nicola CD, Moglie M. A new surface tension equation for refrigerants. Int J Thermophys. 2013;34(12):2243–60.
Zhu J, Duan Y, Yang Z, Lin H. Factors influencing the surface tension of binary hydrocarbon mixtures. Fuel. 2014;116(1):116–22.
Duan W, Zhao X, Zeng X, Liu Y. Surface tension of HFC-161 and compressor oil mixtures. Int J Refrig. 2018;85:191–9.
Carey BS, Scriven LE, Davis HT. Semiempirical theory of surface tensions of pure normal alkanes and alcohols. AIChE J. 1978;24(6):1076–80.
Liang X, Michelsen ML, Kontogeorgis GM. A density gradient theory based method for surface tension calculations. Fluid Phase Equilib. 2016;428:153–63.
Mu X, Frank F, Alpak FO, Chapman WG. Stabilized density gradient theory algorithm for modeling interfacial properties of pure and mixed systems. Fluid Phase Equilib. 2017;435:118–30.
Wang P. Application of green surfactants developing environment friendly foam extinguishing agent. Fire Technol. 2015;51(3):503–11.
Baidakov VG, Khotienkova MN, Andbaeva VN, Kaverin AM. Capillary constant and surface tension of methane-nitrogen solutions: 1. Experiment. Fluid Phase Equilib. 2011;301(1):67–72.
Baidakov VG, Kaverin AM, Khotienkova MN, Andbaeva VN. Surface tension of an ethane-nitrogen solution. 1: experiment and thermodynamic analysis of the results. Fluid Phase Equilib. 2012;328(35):13–20.
Baidakov VG, Kaverin AM, Khotienkova MN. Surface tension of ethane-methane solutions: 1. Experiment and thermodynamic analysis of the results. Fluid Phase Equilib. 2013;356(10):90–5.
Dinenno PJ, Hanauska CP, Forssell EW. Design and engineering aspects of halon replacements. Process Saf Prog. 1995;14(1):57–62.
Yang JC, Pitts WM, Breuel BD, Grosshandler WL, Cleveland WG. Rapid discharge of a fire suppressing agent. Int Commun Heat Mass Transf. 1996;23(23):835–44.
Lemmon EW, Jacobsen RT. A generalized model for the thermodynamic properties of mixtures. Int J Thermophys. 1999;20(3):825–35.
Lemmon EW, Jacobsen RT. Thermodynamic properties of mixtures of R-32, R-125, R-134a, and R-152a. Int J Thermophys. 1999;20(6):1629–38.
He MG, Yang YJ, Zhang Y, Zhang XX. Theoretical estimation of the isobaric heat capacity cp of refrigerant. Appl Therm Eng. 2008;28(14):1813–25.
Hu YQ, Li ZB, Lu JF, Li YG, Jin Y. Surface tension calculation of liquid mixtures by PR EOS. Chem Eng. 1997;25(3):42–5 (in Chinese).
Peng DY, Robinson DB. A new two-constant equation of state. Ind Eng Chem Fundam. 1976;15(1):92–4.
Chen M, Xie Y, Wu H, Shi S, Yu J. Modeling solubility of nitrogen in clean fire extinguishing agent by Peng–Robinson equation of state and a correlation of Henry’s law constants. Appl Therm Eng. 2016;110:457–68.
Holden BS, Katz JL. The homogeneous nucleation of bubbles in superheated binary liquid mixtures. AIChE J. 1978;24(2):260–7.
Lepori L, Gianni P, Matteoli E. Thermodynamic study of tetrachloromethane or heptane + cycloalkane mixtures. J Therm Anal Calorim. 2016;124:1497–509.
Matteoli E, Lepori L, Porcedda S. Thermodynamic study of mixtures containing dibromomethane. J Therm Anal Calorim. 2018;132:611–21.
Chen M, Xie Y, Guo X, Yu J, Ma W. Predicting filling mass of nitrogen in fire agent bottle based on Peng–Robinson equation of state with Wong-Sandler mixing rule. J Beijing Univ Aeron Astron. 2016;42(10):2162–7 (in Chinese).
Simoiu L, Trandafir I, Popescu G. New thermodynamic consistency test for isobaric vapour–liquid equilibrium data. J Therm Anal Calorim. 1998;52:1023–35.
Acknowledgements
The authors would like to acknowledge the financial support from University of Hertfordshire, UK. This work was supported by China Helicopter Design and Research Institute.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, S., Xie, Y., Chen, M. et al. Prediction of the release process of the nitrogen-extinguishant binary mixture considering surface tension. J Therm Anal Calorim 145, 185–199 (2021). https://doi.org/10.1007/s10973-020-10040-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10040-2