Abstract
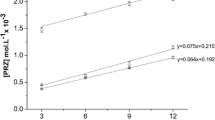
Aripiprazole (ARP) is one of the newest antipsychotic drugs, exhibiting very low aqueous solubility and high lipophilicity. Considering the necessity of improvement of ARP physicochemical properties and its biopharmaceutical profile, cyclodextrin complexation of the drug substance was performed. As selected cyclodextrin, a functionalized β-cyclodextrin was used, namely heptakis(2,6-di-O-methyl)-β-cyclodextrin (DIMEB), and the supramolecular adduct ARP/DIMEB was prepared by kneading technique and characterized using thermoanalytical tools (TG—thermogravimetry/DTG—derivative thermogravimetry/HF—heat flow), powder X-ray diffractometry patterns (PXRD), universal-attenuated total reflectance Fourier transform infrared (UATR-FTIR) and UV (ultraviolet) spectroscopy and, as well, saturation solubility studies. Job’s method was used for the stoichiometry of APR/DIMEB inclusion complex determination, which was found to be 1:2. Molecular modeling studies were complementary realized as to get a view over the way that ARP is hosted inside the cyclodextrin. The compatibility between the inclusion complex and some common pharmaceutical excipients, namely starch, magnesium stearate, lactose monohydrate, microcrystalline cellulose and methylcellulose, has been evaluated by means of thermal methods of analysis (TG/DTG/HF), UATR-FTIR spectroscopy and PXRD pattern. The preformulation data regarding the compatibility of ARP/DIMEB complex with selected excipients suggested that under ambient conditions, chemical interactions are observed solely between ARP/DIMEB inclusion complex and magnesium stearate, as indicated by the UATR-FTIR spectroscopy. The incompatibility in the system ARP/DIMEB + MgS is also confirmed by the PXRD study, this second investigational technique revealing also the advanced amorphization of components during mixing of complex with starch, microcrystalline cellulose and methylcellulose, but without indicating interactions. Later on, under thermal stress, thermally induced interactions occur between the components in the systems containing magnesium stearate and methylcellulose, while starch, microcrystalline cellulose and lactose can be safely used as excipients in developing solid formulations containing ARP/DIMEB inclusion complex as active pharmaceutical ingredient.










Similar content being viewed by others
References
Magliocco F, de Filippis R, Aloi M, Staltari FA, Gaetano R, Segura-Garcia C, De Fazio P. Second-generation long-acting injections anti-psychotics improve executive functions in patients with schizophrenia: a 12-month real-world study. Int J Psychiatry Clin Pract. 2020. https://doi.org/10.1080/13651501.2020.1737134.
Azorin JM, Simon N. Dopamine receptor partial agonists for the treatment of bipolar disorder. Drugs. 2019;79(15):1657–77.
Simon N, Azorin JM. Aripiprazole as dopamine partial agonist model: basic concepts and clinical impact. Encephale. 2018;44(6):558–64.
Saxena K, Kurian S, Saxena J, Goldberg A, Chen E, Simonetti A. Mixed states in early-onset bipolar disorder. Psychiatr Clin North Am. 2020;43(1):95–111.
Censi R, Di Martino P. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules. 2015;20(10):18759–76.
Wu Y, Cheng L, Wu J, Zhai M, Cai L. Cosolvent effect on solubility of aripiprazole in mixed solvents and apparent thermodynamic analysis of dissolution process. J Chem Thermodyn. 2019;135:330–5.
Ardiana F, Lestari MLAD, Indrayanto G. Chapter two—aripiprazole. In: Brittain HG (ed) Profiles drug subst. excip. relat. methodol. Academic Press, pp 35–85.
Brittain HG. Aripiprazole: polymorphs and solvatomorphs. Profiles Drug Subst Excip Relat Methodol. 2012;37:1–29.
Tan X, Zhong Y, He L, Zhang Y, Jing G, Li S, Wang J, He H, Tang X. Morphological and crystalline transitions in monohydrous and anhydrous aripiprazole for a long-acting injectable suspension. AAPS Pharm Sci Tech. 2017;18(4):1270–6.
Zeidan TA, Trotta JT, Chiarella RA, Oliveira MA, Hickey MB, Almarsson Ö, Remenar JF. Polymorphism of dehydro-aripiprazole, the active metabolite of the antipsychotic drug aripiprazole (abilify). Cryst Growth Des. 2013;13(5):2036–46.
Braun DE, Gelbrich T, Kahlenberg V, Tessadri R, Wieser J, Griesser UJ. Stability of solvates and packing systematics of nine crystal forms of the antipsychotic drug aripiprazole. Cryst Growth Des. 2009;9(2):1054–65.
Łyszczarz E, Hofmanová J, Szafraniec-Szczęsny J, Jachowicz R. Orodispersible films containing ball milled aripiprazole-poloxamer®407 solid dispersions. Int J Pharm. 2020. https://doi.org/10.1016/j.ijpharm.2019.118955.
Ramya AR, Sudheer P, Mohameid AS, Das AK. Design and evaluation of a self-emulsifying drug delivery system of aripiprazole. Indian J Pharm Sci. 2019;81(6):1089–98.
McFall H, Sarabu S, Shankar V, Bandari S, Murthy SN, Kolter K, Langley N, Kim DW, Repka MA. Formulation of aripiprazole-loaded pH-modulated solid dispersions via hot-melt extrusion technology: in vitro and in vivo studies. Int J Pharm. 2019;554:302–11.
Nanubolu JB, Ravikumar K. Correlating the melting point alteration with the supramolecular structure in aripiprazole drug cocrystals. Cryst Eng Comm. 2016;18(6):1024–38.
Abdelbary AA, Li X, El-Nabarawi M, Elassasy A, Jasti B. Comparison of nanomilling and coprecipitation on the enhancement of in vitro dissolution rate of poorly water-soluble model drug aripiprazole. Pharm Dev Technol. 2014;19(4):491–500.
Fernández Casares A, Nap WM, Ten Figás G, Huizenga P, Groot R, Hoffmann M. An evaluation of salt screening methodologies. J Pharm Pharmacol. 2015;67(6):812–22.
Oh YJ, Choi G, Choy Bin Y, Park JW, Park JH, Lee HJ, Yoon YJ, Chang HC, Choy JH. Aripiprazole-montmorillonite: a new organic–inorganic nanohybrid material for biomedical applications. Chem Eur J. 2013;19(15):4869–75.
Mihajlovic T, Kachrimanis K, Graovac A, Djuric Z, Ibric S. Improvement of aripiprazole solubility by complexation with (2-hydroxy)propyl-β-cyclodextrin using spray drying technique. AAPS Pharm Sci Tech. 2012;13(2):623–31.
Badr-Eldin SM, Ahmed TA, Ismail HR. Aripiprazole-cyclodextrin binary systems for dissolution enhancement: effect of preparation technique, cyclodextrin type and molar ratio. Iran J Basic Med Sci. 2013;16(12):1223–31.
Tănase IM, Sbârcea L, Ledeți A, Vlase G, Barvinschi P, Văruţ RM, Dragomirescu A, Axente C, Ledeți I. Physicochemical characterization and molecular modeling study of host–guest systems of aripiprazole and functionalized cyclodextrins. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09549-3.
Trandafirescu C, Ledeţi I, Şoica C, Ledeţi A, Vlase G, Borcan F, Dehelean C, Coricovac D, Racoviceanu R, Aigner Z. Albendazole-cyclodextrins binary systems: thermal and spectral investigation on drug-excipient interaction. J Therm Anal Calorim. 2019;138(5):3039–54.
Circioban D, Ledeti A, Vlase G, Moaca A, Ledeti I, Farcas C, Vlase T, Dehelean C. Thermal degradation, kinetic analysis and evaluation of biological activity on human melanoma for artemisinin. J Therm Anal Calorim. 2018;134(1):741–8.
Kim DH, Lee SE, Pyo YC, Tran P, Park JS. Solubility enhancement and application of cyclodextrins in local drug delivery. J Pharm Investig. 2020;50(1):17–27.
D’Aria F, Serri C, Niccoli M, Mayol L, Quagliariello V, Iaffaioli RV, Biondi M, Giancola C. Host–guest inclusion complex of quercetin and hydroxypropyl-β-cyclodextrin: a calorimetric study. J Therm Anal Calorim. 2017;130(1):451–6.
de Lima Ramos Júnior FJ, da Silva KMA, Brandão DO, et al. Investigation of the thermal behavior of inclusion complexes with antifungal activity. J Therm Anal Calorim. 2018;133(1):641–8.
Ohata T, Ikeda H, Mizobe T, Yukawa M, Aki H. Effect of solution pH on complex formation between epi-type catechin and β-cyclodextrin. J Therm Anal Calorim. 2019;135(5):2837–41.
Ikeda H, Ohata T, Yukawa M, Fujisawa M, Aki H. Difference in formation mechanism of inclusion complex between configuration isomers of gallate-type catechin and β-cyclodextrin. J Therm Anal Calorim. 2019;135(5):2789–95.
Pires FQ, Pinho LA, Freire DO, Silva ICR, Sa-Barreto LL, Cardozo-Filho L, Gratieri T, Gelfuso GM, Cunha-Filho M. Thermal analysis used to guide the production of thymol and Lippia origanoides essential oil inclusion complexes with cyclodextrin. J Therm Anal Calorim. 2019;137(2):543–53.
Usacheva T, Kabirov D, Beregova D, Gamov G, Sharnin V, Biondi M, Mayol L, D’Aria F, Giancola C. Thermodynamics of complex formation between hydroxypropyl-β-cyclodextrin and quercetin in water–ethanol solvents at T = 298.15 K. J Therm Anal Calorim. 2019;138(1):417–24.
Sbârcea L, Ledeţi A, Udrescu L, Văruţ RM, Barvinschi P, Vlase G, Ledeţi I. Betulonic acid—cyclodextrins inclusion complexes. J Therm Anal Calorim. 2019;138(4):2787–97.
Oliva R, Battista F, Cozzolino S, Notomista E, Winter R, Del Vecchio P, Petraccone L. Encapsulating properties of sulfobutylether-β-cyclodextrin toward a thrombin-derived antimicrobial peptide. J Therm Anal Calorim. 2019;138(5):3249–56.
Abilify. https://www.drugs.com/pro/abilify.html. Accessed 13 April 2020.
Zhang L, Luan H, Lu W, Wang H. Preformulation studies and enabling formulation selection for an insoluble compound at preclinical stage—from in vitro, in silico to in vivo. J Pharm Sci. 2020;109(2):950–8.
Noonan TJ, Chibale K, Bourne SA, Caira MR. A preformulation co-crystal screening case study: polymorphic co-crystals of an imidazopyridazine antimalarial drug lead with the coformer succinic acid. J Mol Struct. 2020. https://doi.org/10.1016/j.molstruc.2019.127561.
Rathod VR, Shah DA, Dave RH. Systematic implementation of quality-by-design (QbD) to develop NSAID-loaded nanostructured lipid carriers for ocular application: preformulation screening studies and statistical hybrid-design for optimization of variables. Drug Dev Ind Pharm. 2020;46(3):443–55.
Kacso I, Rus LM, Martin F, Miclaus M, Filip X, Dan M. Solid-state compatibility studies of ketoconazole-fumaric acid co-crystal with tablet excipients. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09340-4.
Arnfast L, van Renterghem J, Aho J, Bøtker J, Raijada D, Baldursdóttir S, De Beer T, Rantanen J. Exploring the complexity of processing-induced dehydration during hot melt extrusion using in-line Raman spectroscopy. Pharmaceutics. 2020. https://doi.org/10.3390/pharmaceutics12020116.
Ledeți I, Romanescu M, Cîrcioban D, et al. Stability and compatibility studies of levothyroxine sodium in solid binary systems—instrumental screening. Pharmaceutics. 2020;12(1):58.
Sbârcea L, Ledeţi I, Drəgan L, Kurunczi L, Fuliaş A, Udrescu L. Fosinopril sodium-hydroxypropyl-β-cyclodextrin inclusion complex: thermal decomposition kinetics and compatibility studies. J Therm Anal Calorim. 2015;120(1):981–90.
Udrescu L, Sbârcea L, Fuliaş A, Ledeţi I, Vlase G, Barvinschi P, Kurunczi L. Physicochemical analysis and molecular modeling of the Fosinopril β-cyclodextrin inclusion complex. J Spectrosc. 2014. https://doi.org/10.1155/2014/748468.
Benesi HA, Hildebrand JH. A Spectrophotometric Investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc. 1949;71(8):2703–7.
Srinivasan K, Stalin T, Sivakumar K. Spectral and electrochemical study of host-guest inclusion complex between 2,4-dinitrophenol and β-cyclodextrin. Spectrochim Acta Part A Mol Biomol Spectrosc. 2012;94:89–100.
Protein Data Bank. http://www.pdb.org/pdb/home/home.do. Accessed 15 November 2019.
DeLano WL. PyMOL, San Carlos, CA, 700, 2002.
Job P. Formation and stability of inorganic complexes in solution. Ann di Chim Appl. 1928;9:113–203.
Sbârcea L, Udrescu L, Ledeţi I, Szabadai Z, Fuliaş A, Sbârcea C. β-Cyclodextrin inclusion complexes of lisinopril and zofenopril: physicochemical characterization and compatibility study of lisinopril-β-cyclodextrin with lactose. J Therm Anal Calorim. 2016;123(3):2377–90.
Sbârcea L, Udrescu L, Drǎgan L, Trandafirescu C, Szabadai Z, Bojiţǎ M. Fosinopril-cyclodextrin inclusion complexes: phase solubility and physicochemical analysis. Pharmazie. 2011;66(8):584–9.
Doile MM, Fortunato KA, Schmücker IC, Schucko SK, Silva MAS, Rodrigues PO. Physicochemical properties and dissolution studies of dexamethasone acetate-β-cyclodextrin inclusion complexes produced by different methods. AAPS Pharm Sci Tech. 2008;9(1):314–21.
Circioban D, Ledeti A, Vlase G, Coricovac D, Moaca A, Farcas C, Vlase T, Ledeti I, Dehelean C. Guest–host interactions and complex formation for artemisinin with cyclodextrins: instrumental analysis and evaluation of biological activity. J Therm Anal Calorim. 2018;134(2):1375–84.
Mennini N, Maestrelli F, Cirri M, Mura P. Analysis of physicochemical properties of ternary systems of oxaprozin with randomly methylated-ß-cyclodextrin and l-arginine aimed to improve the drug solubility. J Pharm Biomed Anal. 2016;129:350–8.
Huey R, Morris GM, Olson AJ, Goodsell DS. A semiempirical free energy force field with charge-based desolvation. J Comput Chem. 2007;28(6):1145–52.
Baka E, Comer JEA, Takács-Novák K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J Pharm Biomed Anal. 2008;46(2):335–41.
Udrescu L, Sbârcea L, Fuliaş A, LedeţI I, Vlase T, Barvinschi P, Kurunczi L. Physicochemical characterization of zofenopril inclusion complex with 2-hydroxypropyl-β-cyclodextrin. J Serbian Chem Soc. 2015;80(4):485–97.
Udrescu L, Fuliaş A, Ledeţi I, Vlase G, Barvinschi P, Kurunczi L, Sbarcea L. Host-guest system of zofenopril and randomly methylated β-cyclodextrin. Rev Chim. 2015;66(1):17–20.
Rus LM, Iurian S, Kacso I, Borodi G, Porav S, Hegheş SC, Iuga CA, Tomuţă I. Development of meloxicam oral lyophilisates: role of thermal analysis and complementary techniques. Farmacia. 2019;67(1):56–67.
Cristea M, Baul B, Ledeţi I, et al. Preformulation studies for atorvastatin calcium: an instrumental approach. J Therm Anal Calorim. 2019;138(4):2799–806.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tănase, IM., Sbârcea, L., Ledeţi, A. et al. Compatibility studies with pharmaceutical excipients for aripiprazole–heptakis (2,6-di-O-methyl)-β-cyclodextrin supramolecular adduct. J Therm Anal Calorim 142, 1963–1976 (2020). https://doi.org/10.1007/s10973-020-09901-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09901-7