Abstract
The review presents the results of the investigations of the thermal properties of selected II–VI binary, ternary, and quaternary semiconductors. All tested crystals were grown from the melt using the high-pressure vertical Bridgman–Stockbarger method with different composition The main objective of the work was systematic examination of the thermal properties of the crystals using contact (PPE photopyroelectric) and non-contact (active thermography) measurement techniques. As a result of the studies, the values of the thermal diffusivity and the effusivity of all tested samples were obtained. For the selected series of crystals, their heat capacity was also determined. The thermal conductivity was calculated using simple relationships that combine all the thermal parameters. In this way, a complete thermal characterization of the crystals was carried out. For several ternary mixed crystals diagrams of the thermal conductivity versus composition were analyzed applying model for mixed semiconducting crystals given by Sadao Adachi. Thanks to that a contribution of the thermal resistivity arising from the lattice disorder to the total resistivity of the crystal has been determined. Although the PPE method itself is fairly simple, each material class requires a different approach. For samples with strongly different conductivity or thickness, different frequency ranges and a suitable detector should be used. This is not a simple task for the samples with unknown thermal properties. It is to mention that the PPE technique is a contact method and its main disadvantage in case of solid samples is to provide good thermal contact between the sample and the detector. In practice, it is realized by gluing a sample to the detector with a small amount of different types of liquids. It has been shown that the influence of the coupling layer can be minimized by appropriate modification of the experimental system and proper selection of the coupling fluid. In this way, the photopyroelectric method has been satisfactorily adapted for the thermal characterization of II–VI crystals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Broadband solid solutions of II–VI compounds are very interesting semiconductors for both cognitive and application reasons. Their physical properties are poorly examined mainly because of the difficult technology of their production. These materials have been used as potential light sources [1], as magnetic materials [2], as infrared, ultraviolet, x and gamma ray detectors [3,4,5], in photovoltaics [6], as ZnO-based devices [7], and in other modern optoelectronics applications. From an application point of view, the very important feature of mixed ternary and quaternary crystals is the possibility of an almost constant change of the value of their energy gap and lattice constant.
For many optoelectronic devices, proper heat dissipation is critical for correct functioning. Therefore one of the most important parameters characterizing utility materials, especially materials used in electronics, is the thermal conductivity. The phenomenon of the thermal conductivity of the crystals is a complex issue. Both phonons (crystalline lattice) and free carriers can be involved in the thermal conductivity. However, in the case of II–VI semiconductors, the contribution from free carriers is practically negligible [8]. The key issue in the thermal conductivity is the rigidity of the crystalline lattice and the number of scattering centers of phonons. Because of the high sensitivity of the thermal conductivity to the quality of the crystalline structure, its measurement gives a straightforward information on the quality of the material being tested. In turn, the inverse of the thermal diffusivity [9], called the temperature equalization coefficient, is a very important parameter from the application point of view because is needed in the design and construction of semiconductor devices. Determining the thermal parameters of completely new materials is therefore very important.
All investigated crystals within this work were obtained by modified vertical Bridgman method on the basis of binary high purity compounds such as ZnSe, ZnTe, CdSe and CdTe. The main purpose of this work was to investigate the dependence of the thermal parameters on the composition for individual series of ternary and quaternary crystals. Within the scope of this work, the thermal parameters of over 50 different types of mixed crystals of II–VI groups have been characterized. These were the following groups of materials: binary ZnSe, ZnTe, CdSe and CdTe, ternary: Zn1−xMgxSe, Zn1−xBexSe, Cd1−xMnxTe, Cd1−xZnxTe and quaternary: Cd1−x−yZnxMgySe, Zn1−x−yBexMnySe, Zn1−x−yBexMgySe. All of these compounds are or may be used in modern optoelectronic devices such as lasers, light-emitting diodes, or detectors.
The photopyroelectric calorimetry method in different experimental configuration was used in order of determining the thermal parameters of the investigated crystals [10,11,12,13]. In this way, the thermal diffusivity and the effusivity of the tested materials were determined. In order to calculate the specific heat of the test compounds, their density was determined by geometric and mass measurements. Finally, from the simply dependencies between thermal parameters, the thermal conductivity was obtained. In this way, the main purpose of the work was fulfilled, which was the complete thermal characterization of selected II–VI crystals as the function of the composition. The obtained results were analyzed using model proposed by Adachi for mixed semiconductor crystals [14, 15].
The PPE technique is a contact method and its main disadvantage in case of solid samples is to provide good thermal contact between the sample and the detector [16,17,18]. In practice, it is realized by gluing a sample to the detector with a small amount of different types of liquids. The obtained results are usually controlled by various non-contact methods, like Contactless
Photo Thermal Infrared Radiometry [19] or Laser Flash Analysis [20]. In this work, the author verified the obtained results using active infrared thermography with a lock-in detection [21, 22]. To realize the goal of this work, it was necessary to adapt the PPE method in the case of II–VI semiconductors. It has been shown that the influence of the coupling layer can be minimized by appropriate modification of the experimental system and proper selection of the coupling fluid. In this way, the photopyroelectric method has been satisfactorily adapted for the thermal characterization of II–VI semiconductors.
Theoretical background of applied measurement techniques
PPE method is based on the pyroelectric detection, where the sample-detector system is excited by intensity-modulated radiation in two basic configurations (Fig. 1). In the case where the light falls directly on the sample, we are dealing with a back configuration (BPPE), otherwise the configuration is called the front (FPPE).
Back PPE configuration
In the BPPE configuration, sample s is placed on the pyroelectric detector p and whole system is surrounded by air. Assuming a perfect thermal contact between the sample and the sensor and the one-dimensional model of the heat propagation through the sandwich-like system, the complex PPE signal in back configuration is given by [10,11,12]:
where V0 is an instrumental factor, ij represents s and p layers of the detection cell, respectively, Rij = (bij – 1)/(bij + 1) is the reflection coefficient of the thermal wave at ij interface, bij= ei/ej and e is the thermal effusivity, σi= (1 + i)ai is the complex diffusion coefficient, ai is the reciprocal of the thermal diffusion length μi, ai = 1/μi, μi = (2αi/ω)1/2, ω is the angular modulation frequency and Li is the thickness of the layer i. In order to eliminate the instrumental factorm V0 was eliminated applying empty sensor normalization procedure [12]. Assuming thermally thick regime for both the detector and the sample (μi ≪ Li, the thermal wave is attenuated at least e times on the distance equals of the thickness of the sample), the complex signal can be separate into the amplitude (2) and the phase (3):
The value of the thermal diffusivity can be determined using both the amplitude and the phase of the pyroelectric signal. For this purpose, measurement should be made as a function of the thickness of the test specimen or the modulation frequency. The first type of the measurement is possible only for liquids, in the case of solids a frequency scan is needed. The thermal diffusivity values presented in this work were obtained from the slope n of the phase plot as a function of the square root of the modulation frequency. In this case, the thermal diffusivity can be calculated according to the following formula:
The results obtained from the amplitude were only considered as reference, i.e.in case of the large difference between amplitude and phase, the measurement was rejected as incorrect. The amplitude can be affected by external factors such as laser-intensity fluctuations and the roughness of the surface, whereas the phase is being independent on these external factors. For this reason, the thermal diffusivity was calculated according to Eq. (4).
In the above formulas, the ideal thermal contact between the detector and the test sample is assumed, omitting the effect of the coupling layer. In fact, the obtained thermal diffusivity values are always more or less affected by the additional uncertainty resulting from the need of a coupling liquid. In order to analyze this problem, numerous theoretical simulations based on the 5-layer model were done (Fig. 2).
Theoretical calculations were performed on the basis of the complex equation describing such system, which was given by Salazar [17]:
where Vn is the normalized pyroelectric signal, Ri is is the optical reflection coefficient, indexes s, f and p refer to the opaque sample, fluid and pyroelectric sensor, respectively.
Front PPE configuration
In the front configuration, the excitation light falls directly on the pyroelectric sensor, while the sample placed on the opposite site acts as a heat sink. The complex pyroelectric signal after normalization (using the empty sensor procedure) in this case is given by the following formula [10,11,12]:
Assuming a thermally thick the detector and the sample, we get:
It can be shown that in this case amplitude Vn and phase ϕn of the pyroelectric signal can be expressed as [12]:
The thermal effusivity of the test sample can be obtained by matching the aforementioned theoretical relationships to the experimental points. If one knows the thermal parameters of the test sample, it is possible to determine the thermal properties of the detector itself. For the normalized phase using the empty sensor procedure, the following relationship is true [12]:
where fo is the frequency, where the phase is crossing zero and becomes negative. This formula enables determining the thermal diffusivity of the detector.
The disadvantage of the PPE technique especially in the back, but also in the front configuration is the need to use a liquid coupling the sample with the detector. Ensuring a perfect thermal contact in practice is very difficult task. Therefore, a thermal wave resonator cavity (TWRC) technique has been developed [12, 23]. In this method, the measuring cell consists of a detector, a coupling liquid, a separator and a back layer (Fig. 3).
In the approximation of a one-directional heat propagation the normalized complex PPE signal is given by:
where
If the backing material is air and in the same time L3 is thermally thick (exp(− 2σ3L3) ~ 0) ρ43 does not count and ρ32 is reduced to (a separator layer becomes the backing): ρ32 = (1 – b32)/(1 + b32). The main advantage of this configuration, compared to the classical frequency scanning methods, is connected with the possibility of controlling the type and the thickness variation of the coupling fluid [12]. Moreover, no additional normalization measurement is required, the normalization signal is contained in the same scanning run (thermally very thick regime for the scanned liquid). Basically, in this configuration, one can get the thermal parameters of each layer of the detection cell (if the thermal parameters of the other layers are known). In this work one focused only on the thermal effusivity of the sample inserted as the backing in the detection cell. The disadvantage of this technique compared to the standard measurement in the front configuration is the higher degree of complexity of the measuring system and more time-consuming measurement.
An active infrared thermography
As mentioned above, the need of using of coupling liquid in a pyroelectric method introduces the uncertainty associated with the quality of the thermal contact. For this reason, non-contact methods for the determination of the thermal diffusivity are often used to verify obtained results. In this work, an active infrared thermography with a suitable camera with built-in lock-in detection was used. The theoretical basis of thermography for determining the thermal diffusivity of the tested samples is shown below.
The thermal wave propagating along the surface generated by a time-periodic punctual heat source in an isotropic, homogeneous and thermally thick medium with the thermal diffusivity α, can be written as [21, 24]:
where x is the thermal wave propagation direction, T0 is the surface temperature, f is the excitation frequency, t is the time and k is the wave vector. The 1-D thermal diffusion equation is then given by:
where
For physical reasons, the thermal wave must converge at infinity, which requires:
The thermal wave can be written as follows:
The propagation of a plane thermal wave through a medium of the thickness x and the thermal diffusivity α products a phase shift ∆φ having the following expression:
where a is the slope of the phase-distance graph. The thermal diffusion length is expressed by:
The thermal diffusivity of the tested sample can be calculated according to the above equation.
Description of the model given by Adachi
During measurements the thermal diffusivity and the effusivity of the investigated crystals were obtained. It is known that all thermal parameters are related to each other. The thermal diffusivity is given by a simple formula:
where k is the thermal conductivity, ρ is the density and C is the specific heat capacity. On the other hand, the thermal effusivity can be expresses as:
Using both expressions one can eliminate ρC factor and calculate then the thermal conductivity from simply relation:
In this way, the investigated in this work samples have been characterized for their thermal properties. In the case of II–VI semiconductors the main contribution to the thermal conductivity is related with a phonon mechanism [8]. Lattice thermal conductivity in the case of semiconductor alloys requires taking into account a contribution, which is the result of a random distribution of constituent atoms in sublattice sites. A phenomenological model of the lattice thermal conductivity for semiconductor alloys was first proposed by Abeles [14]. However, Adachi [15] showed that the thermal resistivity W(x) for ternary AxB1−xC alloy can be described by such simple expression:
where WAC and WBC are binary thermal resistivities and CA–B is a contribution arising from the lattice disorder. Equation (22) is easily transferred into lattice thermal conductivity K(x):
In this work, the results obtained for the selected ternary semiconductor alloys have been analyzed within the above model.
Samples and experimental systems
All the crystals, which were used for the study, were grown by high-pressure and high-temperature modified Bridgman method [25]. The growth processes were carried out under typical argon pressure of about 150 atm. and allowed obtaining crystals a few centimeters in the length and about 1 cm in the diameter. Such prepared crystals were next cut into about 1.5 mm thick plates. One can obtain up to 15–18 plates, depending on their thickness and the length of the grown crystal rod. The samples after grounding (Al2O3 powder, 10 μm in diameter) were polished with fine polishing powder (1 μm in diameter). The real composition of the prepared specimens was determined primarily by characteristic radiation spectroscopy using the Quantax 200 spectrometer and the EDX XFlash 4010 detector. Such prepared samples were used for the measurements. Example EDX spectra of the Cd1−xZnxTe crystals under 20 keV excitation with the cutting scheme visible in the inset are presented in Fig. 4.
The composition of the grown crystals was determined every third plate for all Cd1−xZnxTe alloys. For each sample the analysis was done at three different places. The obtained real compositions presented within this work are average values given in atomic percent of zinc content in Cd1−xZnxTe crystals. The uncertainty of the method in this case was of about 2% of the measured value. The more detailed information concerning the growth and the composition of the Cd1−xZnxTe alloys can be found in the Ref. [26]. Also some XRD data for Cd1−xZnxTe mixed crystals can be found here [27].
The pyroelectric detection system, both in the back and front configurations, consisted of a pyroelectric detector (LiTaO3 crystal plates coated with chromium and gold on both surfaces), two-phase lock-in amplifier (SR830 or SR850) and an exciting laser (532 nm, 200 mW or 405 nm, 300 mW). The laser was electronically modulated via a TTL signal fed from the lock-in amplifier in the frequency range of 1 to tens of Hz. Ethylene glycol was used as a coupling liquid in the both experimental configuration. In the back configuration, a diaphragm was used to prevent accidental illumination of the detector by the exciting radiation. In addition, the optically transparent specimens were covered with a thin layer that absorbed incident radiation. Because the blackening layer was very thin and thermally highly conductive, its influence on the obtained results was neglected. In the TWRC technique, an additional element of the measuring system was a motor that allowed a precise change of the thickness of the coupling fluid.
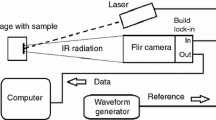
The experimental IR setup included a heat source, a waveform generator, an infrared camera and a computer for data acquisition (Fig. 5). The intensity-modulated optical stimulation was delivered by an Nd:YAG laser (Laser Quantum OPUS, with λ = 532 nm and P = 0.5 W). The IR camera (FLIR 7200 series, with a 256 × 320 pixel array of InSb detectors sensitive in the 1.5–5.1 µm wavelength range, working at a sampling frequency of 100 Hz) recorded the changes in the surface temperature of the specimens.
In the standard BPPE method, a modification was introduced to minimize the influence of the thickness of the coupling layer on the obtained thermal diffusivity values [28, 29]. The modification consisted of pressing the test sample to the sensor by means of a disk and three identical springs acting on the disk. The schema of the altered cell in the back configuration is shown in Fig. 6.
Modified experimental setup for the BPPE method [29]
Discussion of the obtained results
Binary, mixed Cd1−x−yZnxMgySe and Zn1−x−yBexMnySe crystals
The thermal diffusivity of the binary [30], Cd1−x−yZnxMgySe [31] and Zn1−x−yBexMnySe [32] crystals was measured in BPPE configuration and by means of active infrared thermography. In order to compare the obtained results with the literature data, the thermal properties of selected binary II–VI crystals (ZnSe, ZnTe, CdSe and CdTe) were investigated first. In addition, glassy-like carbon GC (type G) with known material properties and a similar thermal diffusivity value compared to the studied semiconductors was used as reference sample. In the case of quaternary crystals, the methodology and measurement techniques were similar to the binary compounds. For this reason, the results of this section will be discussed on the example of binary crystals [30].
Figure 7 presents the typical behavior of the phase as a function of the square root of the modulation frequency for binary crystals and GC sample. For low frequencies, the curves exhibit a nonlinear dependence due to the thermally thin regime of the sample or/and the sensor (thickness 0.5 mm). Starting from f = 4 Hz, the sensor and all samples are thermally thick. In this case, according to theoretical predictions, the phase behaves linearly in the function of the square root of the modulation frequency. Consequently, linear fits have been performed in the frequency range of 4 Hz to 15 Hz, using the least square method. Thermal diffusivities were calculated according to Eq. (4). An active infrared thermography allowed obtaining the thermal phase images of the tested samples. The phase picture obtained for the ZnSe sample (a) with the corresponding phase profiles (b and c) is shown in Fig. 8. The impact zone of the laser is represented by the constant phase zones (plateau on the picture). The thermal wave is propagating continuously, normal to the observed object surface, symmetrically with respect to the excitation point-source.
The BPPE phases in radians of all investigated samples as a function of the square root of the modulation frequency, points are experimental data and lines are linear fits [30]
Thermal image of the phase of the ZnSe a sample and corresponding phase profiles (b, c), circles correspond to measured data and lines are linear fits [30]
The diameter of the laser is about 150μm (1 px = 30 μm). The thermal wave spreads on the surface over a distance of about 3 mm around the excitation source. At this distance from the excitation source, the spherical thermal wave can be approximated by a plane wave. The phase shift + 180° to − 180° is due to the displaying mode after lock-in detection. If such a phase shift occurs, a + 360° phase-correction must be applied. Under coordinate x = 60 px and, respectively, over coordinate x = 260 px (see Fig. 8b, profile 5, for instance), the signal becomes noisy (meaning that the thermal wave is attenuated). The useful signal is between x = 60 and 260 px. The thermal diffusivity was calculated from phase profiles, according to Eq. (18).
The thermal effusivity of the examined crystals was obtained from the TWRC method. Figure 9 presents the results of the phase measurement as a function of the coupling fluid thickness obtained for the ZnSe crystal at two modulation frequencies, 3 and 5 Hz, together with theoretical fittings. According to the theory, two modulation frequencies 3 Hz and 5 Hz were chosen, so the backing sample is thermally thick, and the sensor is still thermally thin (here the thickness of the sensor was 0.2 mm). During the thickness scanning procedure thermal character of the liquid layer is changing from thermally thick to the thin one. In this way the theoretical requirements are being fulfilled. The change of the thermal character of the fluid is reflected in the course of the phase in Fig. 9. For thermally thick regime the phase remains constant (it is used for the normalization) and goes to the minimum when the liquid becomes thermally thin. After the minimum is reached the phase increases very rapidly to its maximum. The higher value of the thermal effusivity of the backing, the wider and deeper minimum is observed as well as the difference between the minimum and maximum values in the phase is increasing.
In order to obtain the sample’s thermal effusivity, numerical calculations have been performed, according to Eq. (11) with two fitting parameters: the effusivity of the backing material (sample) and the value of the absolute thickness of the fluid (distance between the sample and the sensor). The least-square method was applied for the fitting procedure. Thermal properties of the sensor (α = 1.36 × 10−6 m2 s−1 and e = 3660 Ws1/2 m−2 K−1) and ethylene glycol (α = 9.36 × 10−8 m2 s−1 i e = 814 W s1/2 m−2 K−1) were taken from the literature [33].
The best theoretical fits and obtained values of the thermal effusivity are also displayed in Fig. 9. In this way, the thermal effusivity of the tested materials was obtained.
Table 1 contains the thermal parameters of the tested materials. The thermal conductivity was calculated using formula (21). In the case of binary and mixed Cd1−x−yZnxMgySe crystals, their specific heat was calculated in accordance with formula (19). For this purpose, the density of the samples was determined using the assumption of their cylindrical shape and their mass measurements by means of a precise laboratory balance.
In the case of PPE measurements and active IR thermography, the thermal diffusivity measurements for each sample were made three times. The final results presented in Table 1 were averaged over both measurement techniques. Standard deviations are shown only for the selected crystal series. The results of the thermal diffusivity obtained from BPPE were in all cases significantly lower (several percent) than in case of thermography. This observation confirms the influence of the coupling layer on the final results in the case of PPE technique [16,17,18]. The thermal effusivities listed in Table 1 represent the average value of effusivities computed at two excitation frequencies (3 and 5 Hz, respectively). The obtained values lie within 10% deviation of the mean.
The contour maps shown in Fig. 10 represent the precision of the fit performed with Eq. (11) to the experimental data obtained for ZnSe (a) and CdSe (b) crystals, respectively. The x-axis represents the error in the absolute value of the thickness of the coupling fluid. This absolute value is not known, only the thickness variation. The y-axis represents the thermal effusivity. The shape of the contour lines indicates the accuracy of the results. Contour lines extended along Ox axis indicate a good precision in the thermal effusivity measurement. If they extend along Oy axis, the accuracy in the measurement of the thermal effusivity is low. Circles mean similar accuracy in obtaining both the thermal effusivity and location of the backing position. The accuracy of this type of investigations increases if the effusivity ratio coupling fluid/backing material goes to 1. One can see in Fig. 10 that for high backing/liquid (e3/e2) effusivity ratios, an accurate location of the backing material is obtained, while the thermal effusivity is obtained with low precision. This is the case of ZnSe sample, where e3/e2 (5300/890 W s1/2 m−2 K−1) is about 6. For CdSe sample, the ratio is two times smaller and consequently, the precision in the thermal effusivity determination increases.
Table 1 shows that the highest values of the thermal conductivity are observed for binary and mixed crystals with low concentration of added component. Adding even a few percent of the magnesium in any mixed crystal immediately causes a significant deterioration in its thermal properties. The thermal conductivity values differ between the binary ZnSe compound and the mixed crystals Cd1−x−yZnxMgySe or Zn1−x−yBexMnySe even by the order of magnitude. The addition of manganese, beryllium or magnesium generates significant concentrations of various defects in these compounds, which has been confirmed experimentally [37]. Every imperfection of the crystal lattice becomes a potential scattering center for phonons, and consequently the transport capabilities (heat, but also electrical) of such a crystal are greatly reduced. Due to the problem of the heat dissipation in electronic devices, this observation is crucial in the design of new optoelectronic devices based on any mixed compounds.
The results of the thermal conductivity for binary crystals deviate from the literature data [34]. The differences between the values obtained and the literature data observed in this case can be explained partially by a few facts/observations. Thermal properties are much more sensitive to crystal growth techniques than optical parameters. The quality of the grown crystal, its purity, the concentration of defects is crucial here. The studied crystals were obtained by Bridgman’s method, which has the disadvantage of the high electric resistivity of the resulting crystals due to the large concentration of cation vacancies. The obtained values were further influenced by the non-ideal blackening of the samples and the presence of the coupling layer between the sample and the detector.
The thermal conductivity value obtained for the GC reference sample agrees with the data given by the producer [35]. Also, the obtained specific heat value remains in good compliance with the differential scanning calorimetry [36]. By contrast, when comparing literature data for the thermal diffusivity and effusivity, they are respectively under and overestimated.
Observed discrepancies between the literature and measured data prompted the author to look more closely at the used measurement techniques. The results of the optimization studies are presented in the next subsection.
Optimization of the experimental techniques
The effect of the coupling layer on the BPPE results is well known and has also been investigated by other authors [16,17,18]. Salazar et al. have shown that due to the presence of the coupling layer the determined thermal diffusivity of the solid sample is always underestimated [17]. This problem is particularly important for high-conductivity samples and for higher modulation frequencies (above tens of Hz). The proposed solutions of this problem are primarily based on the control of the obtained photopyroelectric results by non-contact methods, such as infrared radiometry (PTR—photothermal radiometry). The author used non-contact active thermography with the lock-in detection. However, the results obtained from the camera may also be subjected to an uncertainty, which is difficult to estimate accurately.
Therefore, in the work [28], the thermal diffusivity measurements of known materials (GC, LiTaO3, CdTe and CdSe) obtained by both mentioned above methods were presented, paying particular attention to fulfill the theoretical assumptions and optimizing the experimental conditions. With the goal to test different coupling fluids in BPPE technique, the thermal diffusivity measurements were made in a standard configuration for G-type (GC) glassy-like carbon for various fluids [28]. The obtained results are presented in Fig. 11.
Phase characteristics of glassy-like carbon for different coupling fluids as a function of the square root of the modulation frequency, points are experimental data and lines are linear fits [28]
The value of the thermal diffusivity of the GC sample given by other authors is in the range of 5.4–6 × 10−6 m2 s−1 [18]. From the above figure, it is clear that less undervalued values were obtained for more liquid substances, with the value obtained for ethylene glycol lying within the error limits of the reference range.
In order to accurately analyze the influence of the thickness of the coupling layer on the obtained thermal diffusivity value, numerous theoretical simulations based on the five-layer model presented in Fig. 2 and Eq. (5) were performed [29].
In Fig. 12 simulations for a hypothetic solid sample with the thermal diffusivity 2 × 10−6 m2 s−1, effusivity 2000 W s1/2 m−2 K−1 and thickness 1 mm for different thickness of the coupling fluid (ethylene glycol) are shown.
Theoretical simulations of the phase of the signal for different thicknesses of the coupling fluid [29]
The thermal parameters of the coupling fluid and the pyroelectric sensor were taken from the literature [38]: for ethylene glycol (αf= 9.36 × 10−8 m2 s−1, ef= 890 W·s1/2·m−2 K−1) and for LiTaO3 (αp= 1.56 × 10−6 m2 s−1, ep= 3600 W·s1/2·m−2 K−1). One can see that the slope a of the curve “phase vs. f1/2” increases from 1.25 to 1.31 with increasing thickness of the coupling fluid from 0 to 10 μm. A similar correspondence “slope of the curve phase vs. f1/2” vs. “coupling fluid thickness” was obtained in the Ref. [17].
The results of the numerical calculation of relative error in the thermal diffusivity evaluation, by applying Eq. (5), for different sets of the parameters, are given in Table 2. The error was calculated as relative value taking into account given thermal diffusivity in comparison with value obtained for zero fluid thickness. The first three rows present the influence of the thickness of the coupling fluid on the value of the thermal diffusivity for a solid sample with low (second row) and higher (third row) the thermal diffusivity. The data presented in Table 2 support our initial statement, that the influence of the coupling medium becomes significant especially for high conductive samples. Relative error for a sample with diffusivity αs= 6 × 10−6 m2 s−1 at a liquid thickness of 10 μm was almost 15%, nearly double that of a weaker-conductive sample. The dependence of the relative error versus the thermal diffusivity ratio (αL/αs) is given in the next two rows of Table 2. As expected, when the values of the thermal diffusivity of the coupling fluid and the sample are close, the error decreases. Last four rows evaluate the influence of the difference between the effusivity of the coupling fluid and that of the solid sample; this difference seems to be not so important like the thickness and the thermal diffusivity of the coupling fluid.
Based on the obtained results, it was concluded that the critical factor in BPPE contact measurement is the thickness of the coupling layer. In the case of liquids, it is easier to obtain a thin, homogeneous layer which gives good thermal contact between the sample and the detector. Ethylene glycol was chosen as the most optimal for further studies. In order to minimize the impact of the ethylene glycol layer, a simple modification to the standard measuring system was introduced, which is shown in Fig. 6. A comparison of experimental results obtained before and after the modification of the experimental setup on the example of CdTe sample is shown in Fig. 13.
Phase characteristics of CdTe sample measured before (squares) and after (circles) the modification of the experimental setup, points—experimental data, lines—linear fits [29]
The difference in the slopes between two curves can be clearly seen, consequently the thermal diffusivity of the specimen was calculated from the slopes as 4.244 ± 0.035 × 10−6 m2 s−1 for standard configuration and as 4.473 ± 0.008 × 10−6 m2 s−1 after applying the modification. Given thermal diffusivities were calculated as average values from three independent measurements with standard deviation as an uncertainty. One can see that thanks proposed simply solution significant reduction of the influence of the coupling fluid takes place (the final thickness of ethylene glycol is reduced). The standard deviation of the thermal diffusivity is smaller in case of modified BPPE technique indicating stable, repeatable measurement conditions.
In the course of further investigations, it was found that the values of the thermal diffusivity obtained by non-contact active infrared thermography are sensitive to the geometry of the measuring system. The test specimen should be mounted as flat-parallel to the lens of the camera. It is not easy to obtain such a setting under testing conditions of measuring system. In the case of inaccurate sample setting with respect to the camera, the slope obtained from the phase profiles (see Fig. 8 for the ZnSe crystal) may differ from each other, generating a relatively high measurement uncertainty that may be several percent of the measured value. Much larger error can generate incorrectly selected experiment conditions. According to the theory, using active thermography, one should work at the modulation frequency for which the sample is thermally thick. Failure to do so may result in an underestimation of the thermal diffusivity of up to 20–30%. Choosing the correct frequency is therefore critical, but it is not an easy task in case of new materials with unknown thermal parameters.
Figure 14 shows the results obtained for the GC sample for different modulation frequencies (0.05–4 Hz). For very low modulation frequency, the sample is thermally thin, and convective effects cannot be ignored. The resulting thermal diffusivity value is too small; for subsequent frequencies the underestimation decreases. For 4 Hz the result is already satisfactory and falls within the range of values reported by other authors. For completely new materials to determine the approximate modulation frequency contact PPE technique can be used first.
Measurements of the thermal diffusivity for glassy-like carbon were performed in the frequency range of 0.05–4 Hz, points refer to experimental data and error bars to the standard deviation [28]
Results of the thermal diffusivity along with standard deviations of GC type G, CdSe, CdTe and LiTaO3 samples measured both by the modified BPPE method and by thermography are presented in Table 3.
Obtained thermal diffusivity values remain in good agreement with literature data given in third column and confirm an ability of applied techniques for proper thermal characterization of solid materials. For CdSe, CdTe and LiTaO3 samples there is a very good agreement between PPE and Thermography methods, obtained values lie within error bars. Some discrepancy of about 4% for glassy-like carbon can be found; however, the difference is not too large. Considering standard deviation, the BPPE technique together with proposed modification is much more stable and accurate than thermography method. However, the photopyroelectric calorimetry is well established technique, in contrast to infrared lock-in thermography, which is quite new technique still under development. From the experimental point of view, infrared lock-in thermography being non-contact method, seems to be an interesting alternative because the measurements are fast and no coupling medium is required.
Cd1−xMnxTe, Zn1−xBexSe, Zn1−xMgxSe, Zn1−x−yBexMgySe and Cd1−xZnxTe compounds
In this subsection, the thermal diffusivity measurements of the tested crystals were made using the modified measuring system shown in Fig. 6 and ethylene glycol as the coupling liquid. In addition, with the exception of Cd1−xMnxTe crystals, the results of the phase measurements are not expressed as a function of the square root of the modulation frequency, but of the square root of the modulation frequency multiplied by the thickness of the sample. Thanks to that the difference in the slopes of obtained curves is directly proportional to the thermal diffusivity of given specimens. The phase characteristics of the thermal diffusivity of crystals Cd1−xZnxTe are shown in Fig. 15 [26].
Phase characteristics of Cd1−xZnxTe crystals for all zinc content, points are experimental data and lines are linear fits obtained with least square method [26]
Linear fits were performed in the range of frequency where both, the sample and the sensor are thermally thick. The statistical determination coefficient R2 describing quality of fitting was better than 0.9999 for all cases. One can see in Fig. 15 that the specimens with the highest and lowest thermal diffusivity (the lowest and highest slope) are ZnTe and Cd0.5Zn0.5Te, respectively. The thermal diffusivity of investigated specimens was calculated according to Eq. (4).
Since in the TWRC method it is difficult to determine the accuracy of the obtained results and due to the complexity of the experiment, the results of the thermal effusivity measurements were obtained in the FPPE configuration. In order to calibrate and test the measuring system the thermal effusivity of distilled water and ethylene glycol was measured first (Fig. 16) [41]. The obtained results were normalized using a standardized empty sensor procedure. The thermal effusivity of the water and ethylene glycol was obtained as the parameter of the fitting of the Eq. (9). Given values were calculated as average values from three independent measurements with standard deviation as an uncertainty. Obtained results of the thermal effusivity of water and ethylene glycol remains in good agreement with the literature data [12]. An identical measurement procedure was used for all semiconductor materials discussed in this section.
Figure 17 presents measured phase of the selected Cd1−xZnxTe crystals in the case of front configuration. For clarity purposes, the results for three selected crystals are only given. The determination coefficient was of the order 0.999, slightly lower than in case of back configuration. The fit of the theoretical curve to the experimental data was done with Eq. (9) applying least square method. The error of the example fitting for Cd0.5Zn0.5Te crystal is given in the inset. The minimum observed in the error graph indicates the sought thermal effusivity value of the investigated sample. One can observe that all curves presented in Fig. 17 cross the zero point more or less at the same modulation frequency. This observation supports the statement about proper measurement procedure.
As in “Binary, mixed Cd1−x−yZnxMgySe and Zn1−x−yBexMnySe crystals” section, the thermal conductivity was calculated from the obtained results. Thermal parameters of the crystals from this section are presented in Table 4 [26, 37, 41].
The thermal diffusivity and effusivity values are given as average from three independent measurements with standard deviation as uncertainty. Uncertainties of the thermal conductivity were calculated using total differential method as simply average errors taking into account thermal diffusivity and effusivity errors. The experimental reproducibility leads to the thermal conductivity uncertainty of the order of 0.5–1.5%, depending on the sample. The thickness of the specimens was measured with a micrometer with an accuracy of 10 μm, which produces additional uncertainty of about 0.2–0.3%. The systematic error connected with the presence of coupling fluid can be estimated as 3–4% [29]. One can conclude that final uncertainty of the thermal conductivity value is of the order of 5–6%. Confirmation of this conclusion is a comparison of the obtained thermal conductivity value of the ZnSe binary compound with the literature data. The author obtained a value of 17.68 W m−1 K−1, slightly less than the literature (19 W m−1 K−1 [29]). However, taking into account the non-ideal experimental conditions as well as the influence of the growth process by various techniques, the difference is not large.
As it was described in “Binary, mixed Cd1−x−yZnxMgySe and Zn1−x−yBexMnySe crystals” section, adding manganese, zinc, magnesium or beryllium to mixed crystals results in a decrease in the value of all thermal parameters. A particularly strong decrease in the thermal conductivity is observed for Zn1−xMgxSe crystals, where conductivity drops from about 18 W m−1 K−1 for pure ZnSe to 2.3 W m−1 K−1 for ZnO.67Mg0.33Se compound. A similar effect is observed in case of crystals with beryllium. The decrease in transportation capacity of the mentioned compounds is due to the increasing concentration of various defects in these crystals. With the concentration of added elements in case of ternary and quaternary compounds, the quality of their crystal structure deteriorates and the disorder of the lattice increases. Analysis of the transport properties of the investigated compounds is not easy task due to the various effects that occur at the time of compound formation. Zn1−xBexSe crystals are a good example here. On the one hand, it is shown that as the beryllium concentration increases, the microhardness increases [25], on the other hand, the lattice disorder is increasing [25, 42]. In turn, the addition of magnesium to the ternary compound Zn1−xBexSe paradoxically improves its transport properties as it has been shown due to compensation effects that reduce the concentration of cation vacancies [42]. This is clearly evident in the results presented in Table 4, where the resulting thermal conductivity of Zn0.72Be0.06Mg0.22Se sample is comparable to the value obtained for the Zn0.74Be0.26Se crystal and even higher than for Zn0.78Mg0.22Se alloy.
The electron contribution to the thermal conductivity in case of wide-gap II–VI crystals is very small indicating that the heat is mainly carried by phonons [8]. Therefore, the thermal conductivity of the crystal can be related only to the lattice contribution. In this case, the approximate formulas (22) and (23) are correct. The thermal conductivity results obtained for the ternary semiconductor crystals Zn1−xBexSe, Zn1−xMgxSe and Cd1−xZnxTe were analyzed in the model given by Adachi. Figure 18 presents the thermal conductivity K(x) as a function of composition at room temperature for selected Zn1−xBexSe alloys. Best fit of Eq. (23) applying least square method was obtained for following parameter values: CZn–Be= 139 W−1 cm K and KBeSe = 1 W cm−1 K−1. Determination coefficient R2 indicating the quality of the fitting procedure was equal 0.9758. Similar results were obtained for Zn1−xMgxSe alloy, where CZn–Mg= 116 W−1 cm K.
Parameters CZn–Be and CZn–Mg describe contribution arising from the lattice disorder and are 2–6 times larger than obtained by Adachi for typical III–V ternary compounds [15]. However, the character of the change of the lattice thermal conductivity is in principal the same. One can observe rapid decrease and increase of the thermal conductivity for x value close to 0 and 1, respectively. Meanwhile in the middle of the graph there is plateau.
Since the experimental data ends at x = 0.26 and due to the nature of the thermal conductivity change in the vicinity of x = 1, it is very difficult to obtain from this data the reliable thermal conductivity value of pure BeSe. This problem is illustrated in Fig. 19, in which the error resulting from the least squares procedure for the two parameters: CZn–Be and kBeSe is shown. While the minimum on the y-axis is well-located, on the x-axis, describing the thermal conductivity of the BeSe compound, is much stretched, making it impossible to determine the value of the kBeSe parameter. The author tested the model given by Adachi for the ability to estimate the value of this parameter. It turned out that at x = 0.8 on the error map the minimum on the y-axis starts to appear. Unfortunately, the melting point of the Zn0.2Be0.8Se compound is beyond capabilities of our crystal growth system. In nature there are no pure BeSe or MgSe binary compounds, also it is very hard to obtain them. Accordingly, there is no experimental data describing the properties of these compounds. However, theoretical predictions regarding the expected values of such materials can be found in the literature. Theoretical calculations indicate the thermal conductivity of beryllium selenide of about 1 W cm−1 K−1, and this value was used in Eq. (23). This value is typical rather for III–V crystals than II–VI, which confirms the atypical properties of mixed Zn1−xBexSe crystals compared to other II–VI compounds. In these crystals, the character of the bond is more covalent [43] than that of other II–VI compounds, where the bonds are of the ionic type. As a result, these crystals are characterized by interesting properties such as small lattice constant or large energy gap.
Contour map of the error from two parameter (CZn–Be and KBeSe) fitting procedure with least square method [37]
For Cd1−xZnxTe crystals, the relationship of the thermal conductivity in the full range of compositions was obtained, i.e. from one binary compound to another (from CdTe to ZnTe) [26]. The results analyzed in the Adachi model are shown in Fig. 20. The points represent the experimental data, the line is the fit of Eq. (23) with the determination coefficient R2= 0.995758.
The character of the thermal conductivity changes in the function of the composition is similar to that of the Zn1−xBexSe and Zn1−xMgxSe crystals. The CCd−Zn coefficient was obtained as 139 W−1 cm K, exactly the same as for the Zn1−xBexSe crystals.
In summary, as can be seen from the three groups of crystal samples, the thermal parameters are very sensitive to the change of composition, especially for the limit values of x lying near 0 and 1. It is possible therefore (after prior calibration) to determine the composition of newly obtained crystals from a given group by measuring their thermal properties and thanks to that also a lattice constant and fundamental energy gap.
Conclusions
The review presents the results of the thermal properties of selected mixed II–VI semiconductors. All tested crystals were grown from the melt using the high-pressure Bridgman method. The first results were obtained for binary, Cd1−x−yZnxMgySe and Zn1−x−yBexMnySe crystals. At this stage, the crystals were examined using both PPE (BPPE and TWRC) contact methods as well as by non-contact active thermography. The highest thermal conductivity values were observed for binary and mixed crystals with low concentration of added component. Adding even a few atomic percent of the foreign cation to any mixed crystal immediately caused a significant deterioration in its thermal properties. The thermal conductivity values differed between the binary ZnSe compound and the mixed crystals Cd1−x−yZnxMgySe or Zn1−x−yBexMnySe even by the order of magnitude. The addition of manganese, beryllium or magnesium generated significant concentrations of various defects in these compounds, and as a consequence, the thermal transport parameters of such crystals have been substantially reduced. Due to the problem of heat dissipation in electronic devices, this observation is crucial in the process of designing new optoelectronic devices based on any mixed compounds.
Particularly important were the results obtained for binary crystals and glassy carbon (GC) samples, as the experimental values were comparable to the literature data. The final results of the thermal conductivity in the case of binary crystals deviated from the literature data. The discrepancy was explained by the effect of the non-ideal blackening layer or/and by the presence of a coupling liquid in the sample/detector system. The thermal conductivity values obtained for the GC reference sample matched the literature data. On the other hand, while comparing literature data on the thermal diffusivity and effusivity of the GC, they were appropriately understated and overvalued.
Observed discrepancies between the literature and measured data prompted the author to look more closely at the used measurement techniques. It has been shown by other authors that the main problem with PPE technique is the effect of the coupling layer, which always influences obtained results. The question was how great the problem is in this particular case, i.e. for the tested material class. In order to analyze this problem, various coupling fluids commonly used in photopyroelectric techniques have been tested first. As the most optimal, i.e. giving the least underestimation, ethylene glycol was chosen which was used in the course of further research. In order to analyze the quantitative influence of the coupling layer on the obtained thermal diffusivity value, simulations were carried out based on a five-layer theoretical model. The results clearly showed that the critical factor in BPPE contact measurement is the thickness of the coupling layer. This effect is further enhanced for high-conductivity samples. In order to minimize the impact of the ethylene glycol layer, a simple modification to the standard measuring system was introduced. The modification consisted of pressing the test sample to the detector by means of a disk and three identical actuating springs, thereby reducing the thickness of the coupling layer. The results obtained with the modified system allowed further reducing of the underestimation to the value of 3–4% of the measured value.
In the case of non-contact active infrared thermography, it turned out that the received thermal diffusivity values are sensitive to the geometry of the measuring system. In the case of inaccurate setting of the sample with respect to the camera, the slopes obtained from the phase profiles differ from each other, generating a relatively high measurement uncertainty of up to a few percent of the measured value. In addition, the measured samples should be thermally thick. Failure to do so results in an underestimation of thermal diffusivity of up to 20–30%. Choosing the right frequency is therefore critical, but it is not an easy task in case of new materials with unknown thermal parameters. For new materials to determine the approximate frequency of the modulation, one can use the contact photopyroelectric method first.
As a result of the optimization processes, the thermal diffusivity values of the reference materials according to the literature data were obtained. The thermal diffusivity measurements of the crystals were made using a modified measuring system and ethylene glycol as the coupling liquid between the sample and the detector. In addition, as in the TWRC method, it is difficult to determine the accuracy of the obtained results and due to the degree of complexity of the experiment, the results of the thermal effusivity measurements were obtained in the standard FPPE configuration. The total uncertainty of the thermal conductivity of the examined crystals was estimated at 5–6% of the measured value. Confirmation of this conclusion was a comparison of the obtained thermal conductivity value of the ZnSe binary compound with the literature data. As with previous crystals, the addition of manganese, zinc, magnesium or beryllium to mixed crystals resulted in a decrease in the value of all thermal parameters. A particularly strong decrease in thermal conductivity was observed for Zn1−xMgxSe crystals, where the conductivity decreased from about 18 W m−1 K−1 for pure ZnSe to 2.3 W m−1 K−1 for ZnO.67Mg0.33Se compound. Similar effects have also been observed for crystals with beryllium. The thermal conductivity results obtained for the ternary semiconductor crystals Zn1−xBexSe, Zn1−xMgxSe and Cd1−xZnxTe were analyzed in a model provided by Adachi. The CZn–Be, CZn–Mg and CCd–Zn parameters (139, 116 and 139 W−1 cm K, respectively) describing the contribution to thermal resistance resulting from the lattice disorder were 2–6 times higher than those obtained by Adachi for typical III–V semiconductors. However, the character of the change of the thermal conductivity in the function of the composition was the same for both classes of materials.
Although the PPE technique itself is fairly simple, each material class requires a different approach. For samples with strongly different conductivity or thickness, different frequency ranges and a suitable detector should be used. This is not a simple task for the samples with unknown thermal properties. However, the results presented in this work indicate that the set objectives have been met. The thermal characterization of the selected II–VI crystals has been carried out. Also, the direct results of the disorder as the function of the composition for selected groups of ternary crystals were presented. And finally, to realize the goal of this work the PPE technique has been successfully adapted for the study of the given material class.
References
Ravikumar AP, Garcia TA, de Jesus J, Tamargo M, Gmachl C. High detectivity short-wavelength II–VI quantum cascade detector. Appl Phys Lett. 2014;105:061113.
Wojnar P, Janik E, Baczewski LT, Kret S, Dynowska E, Wojciechowski T, Suffczyński J, Papierska J, Kossacki P, Karczewski G, Kossut J, Wojtowicz. Giant spin splitting in optically active ZnMnTe/ZnMgTe core/shell nanowires. Nano Lett. 2012;12:3404–9.
Shen A, Ravikumar AP, Chen G, Zhao K, Alfaro-Martinez A, Garcia T, de Jesus J, Tamargo MC, Gmachl C. MBE growth of ZnCdSe/ZnCdMgSe quantum-well infrared photodetectors. J Vac Sci Technol. 2013;31:03C113.
Hou YN, Mei ZX, Liang HL, Ye DQ, Gul CZ, Du XL. Dual-band MgZnO ultraviolet photodetector integrated with Si. Appl Phys Lett. 2013;102:153510.
Niraula M, Yasuda K, Yamashita H, Wajima Y, Tsukamoto Y, Matsumoto M, Suzuki Y, Takai N, Tsukamoto Y, Agata Y. Vapor-phase epitaxial growth of thick single crystal CdTe on Si substrate for X-ray, gamma ray spectroscopic detector development. Phys Status Solidi C. 2014;11:1333–6.
Yu KM, Mayer MA, Speaks DT, He H, Zhao R, Hsu L, Mao SS, Haller EE, Walukiewicz W. Ideal transparent conductors for full spectrum photovoltaics. J Appl Phys. 2012;111:123505-5.
Xiao L, Mei D, Cao M, Qu D, Deng B. Effects of structural patterns and degree of crystallinity on the performance of nanostructured ZnO as anode material for lithium-ion batteries. J Alloy Compd. 2015;2015(627):455–62.
Slack G. Thermal conductivity of II–VI compounds and phonon scattering by Fe2+ impurities. Phys Rev B. 1972;6:3791–9.
Chrobak L, Malinski M, Zakrzewski J. Simultaneous determination of the thermal diffusivity and a drum factor for CdBeMnTe crystals with the photoacoustic method. Thermochim Acta. 2015;606:84–9.
Mandelis A, Zver MM. Theory of photopyroelectric spectroscopy of solids. J Appl Phys. 1985;57:4421–30.
Chirtoc M, Mihailescu G. Theory of the photopyroelectric method for investigation of optical and thermal materials properties. Phys Rev B. 1989;40:9606–17.
Dadarlat D. Photopyroelectric calorimetry of liquids; recent development and applications. Laser Phys. 2009;19:1330–9.
Tripon C, Depriester M, Craciunescu I, Tosa V, Dadarlat D, Sahraoui AH. Photothermal investigations of phase transitions in liquid thermoelectrics. J Therm Anal Calorim. 2019;138:713–20.
Abeles B. Lattice thermal conductivity of disordered semiconductor alloys at high temperatures. Phys Rev. 1963;131:1906–11.
Adachi S. Lattice thermal resistivity of III–V compound alloys. J Appl Phys. 1983;54:1844–8.
Marinelli M, Mercuri F, Zammit U, Pizzoferrato R. The influence of the coupling fluids and of the pyroelectric transducer on low-temperature photopyroelectric studies. Appl Phys A. 1991;52:115–8.
Salazar A. On the influence of the coupling fluid in photopyroelectric measurements. Rev Sci Instrum. 2003;74:825–7.
Salazar A, Oleaga A. Overcoming the influence of the coupling fluid in photopyroelectric measurements of solid samples. Rev Sci Instrum. 2012;83:014903–7.
Chrobak L, Malinski M. On investigations of the optical absorption coefficient of gold and germanium implanted silicon with the use of the non-destructive contactless photo thermal infrared radiometry. J Electron Mater. 2019;48:5273–8.
Kemsies RH, Milkereit B, Lindemann A, Schick C, Kessler O. In situ investigation of precipitation in aluminium alloys via thermal diffusivity from laser flash analysis. J Therm Anal Calorim. 2020;140:725–33.
Boue C, Hole S. Infrared thermography protocol for simple measurements of thermal diffusivity and conductivity. Infrared Phys Technol. 2012;55:376–9.
Cobîrzan N, Thalmaier G, Balog AA, Constantinescu H, Timiş I, Streza M. Thermophysical properties of fired clay bricks with waste ceramics and paper pulp as pore-forming agent. J Therm Anal Calorim. 2018;134:843–51.
Shen J, Mandelis A. Thermal wave resonator cavity. Rev Sci Instrum. 1995;66:4999–5005.
Carslaw HW, Jaeger JC. Conduction of heat in solids. 2nd ed. London: Oxford University Press; 1959.
Firszt F, Łęgowski S, Męczyńska H, Szatkowski J, Paszkowicz W, Godwod K. Growth and characterisation of Zn1−xBexSe mixed crystals. J Cryst Growth. 1998;184(185):1335–7.
Strzałkowski K. The composition effect on the thermal and optical properties across CdZnTe crystals. J Phys D Appl Phys. 2016;49:435106–12.
Hsu HP, Lin DY, Chen CW, Wu YF, Strzałkowski K, Sitarek P. Optical characterizations of Cd1−xZnxTe mixed crystals grown by vertical Bridgman–Stockbarger method. J Cryst Growth. 2020;534:125491–6.
Strzałkowski K, Streza M, Pawlak M. Lock-in thermography versus PPE calorimetry for accurate measurements of thermophysical properties of solid samples: a comparative study. Measurement. 2015;64:64–70.
Strzałkowski K, Dadarlat D, Streza M, Firszt F. On the optimization of experimental parameters in photopyroelectric investigation of thermal diffusivity of solids. Thermochim Acta. 2015;614:232–8.
Strzałkowski K, Streza M, Dadarlat D, Marasek A. Thermal characterization of II–VI binary crystals by photopyroelectric calorimetry and infrared lock-in thermography. J Therm Anal Calorim. 2015;119:319–27.
Strzałkowski K. Characterization of thermal properties of Cd1−x−yZnxMgySe mixed crystals by means of photopyroelectric and infrared imaging techniques. Mater Sci Eng B. 2014;184:80–7.
Strzałkowski K, Dadarlat D, Streza M, Zakrzewski J. Thermal characterization of ZnBeMnSe mixed compounds by means of photopyroelectric and lock-in thermography methods. Appl Phys A. 2015;119:1165–71.
Dadarlat D, Streza M, Pop MN, Tosa V, Delenclos S, Longuemart S, Sahraoui AH. Photopyroelectric calorimetry of FPPE–TWRC method. J Therm Anal Calorim. 2010;101:397–402.
Madelung O. Semiconductors: data handbook. Berlin: Springer; 2003.
http://www.htw-gmbh.de/. Accessed 10 Oct 2019.
Pawlak M, Firszt F, Łegowski S, Meczynska H, Gibkes J, Pelzl J. Thermal transport properties of Cd1-xMgxSe mixed crystals measured by means of the photopyroelectric method. Int J Thermophys. 2010;31:187–98.
Strzałkowski K. Effect of lattice disorder on the thermal conductivity of ZnBeSe, ZnMgSe and ZnBeMgSe crystals. Mater Chem Phys. 2015;163:453–9.
Chirtoc M, Glorieux C, Thoen J. Thermal wave physics and related photothermal techniques: basic principles and recent developments. In: Moares EM, editor. Transworld Research Network. Trivandrum, Kerala, India; 2009. pp. 125.
Coufal H, Mandelis A. Pyroelectric sensors for the photothermal analysis of condensed phases. Ferroelectrics. 1991;118:379–409.
http://www.sttic.com.ru/. Accessed 10 Oct 2019.
Strzałkowski K, Firszt F, Marasek A. Thermal diffusivity, effusivity and conductivity of CdMnTe mixed crystals. Int J Thermophys. 2014;35:2140–9.
Firszt F, Łęgowski S, Męczyńska H, Szatkowski J, Banasiak A, Paszkowicz W, Falke U, Schulze S, Hietschold M. Growth and characterisation of bulk Zn1−xBexSe, Zn1−x−yMgxBeySe and Zn1−xBexTe crystals. J Cryst Growth. 2000;214(215):880–4.
Ganguli T, Mazher J, Polian A, Deb SK, Villain F, Pages O, Paszkowicz W, Firszt F. Lattice relaxation in the highly-contrasted Zn1−xBexSe alloy: an extended x-ray absorption fine structure study. J Appl Phys. 2010;108:083539-1–8.
Acknowledgements
Many people were involved in this work. First of all I would like to thank prof. F. Firszt for many fruitful discussions and any help. All crystals were grown with significant share of A. Marasek, M.Sc. Part of the PPE experiments were done at Laboratory of Dorin Dadarlat, Cluj-Napoca, Romania. The author is also grateful to dr. Mihaela Streza for lock-in thermography measurement. I would like to thank also all my colleagues, dr M. Pawlak and dr. J. Zakrzewski.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Strzałkowski, K. Thermal properties of selected II–VI semiconductors determined by photopyroelectric calorimetry technique. J Therm Anal Calorim 145, 227–244 (2021). https://doi.org/10.1007/s10973-020-09897-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09897-0