Abstract
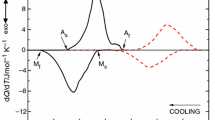
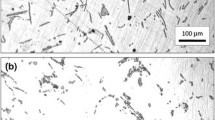
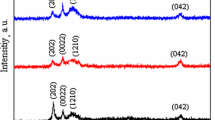
We report data obtained from the spinodal decomposition in samples of two compositions of intermetallic Cu–Al–Mn shape memory alloys. Detailed experimental information has been collected by means of differential scanning calorimetry (DSC). Spinodal decomposition was induced by isothermal treatments at different temperatures within the miscibility gap. To determine the precipitation kinetics, after each isothermal treatment particle dissolution curves were obtained by DSC. From the dissolution curves, various aspects of the precipitation process were determined, such as particles dissolution enthalpy; initial precipitate volume fraction evolution; non-isothermal dissolution characteristics; dissolution activation energy and particle distribution information. Regardless of the composition, the determined activation energy was 77 kJ mol−1. Diffusion of Mn atoms seems to be the fundamental factor that controls the studied processes.







Similar content being viewed by others
References
Kainuma R, Satoh N, Liu XJ, Ohnuma I, Ishida K. Phase equilibria and Heusler phase stability in the Cu-rich portion of the Cu–Al–Mn system. J Alloys Compd. 1998;266:191–200.
Kainuma R, Takahashi S, Ishida K. Thermoelastic martensite and shape memory effect in ductile Cu–Al–Mn alloys. Metall Mater Trans A. 1996;27:2187–95.
Sutou Y, Koeda N, Omori T, Kainuma R, Ishida K. Effects of ageing on bainitic and thermally induced martensitic transformations in ductile Cu–Al–Mn-based shape memory alloys. Acta Mater Acta Mater Inc. 2009;57:5748–58.
Brazolin GF, Silva CCS, Silva LS, Silva RAG. Phase transformations in an annealed Cu–9Al–10Mn–3Gd alloy. J Therm Anal Calorim. 2018;134(3):1405–12.
Souza JS, Modesto DA, Silva RAG. Thermal behavior of the as-cast Cu–11Al–10Mn alloy with Sn and Gd additions. J Therm Anal Calorim. 2019;7:1–8.
Obradó E, Frontera C, Mañosa L, Planes A. Order-disorder transitions of Cu–Al–Mn shape-memory alloys. Phys Rev B. 1998;58:14245–55.
Prado MO, Decorte PM, Lovey FC. Martensitic transformation in Cu–Mn–Al alloys. Scr Metall Mater Mater. 1995;33:877–83.
Bouchard M, Thomas G. Phase transitions and modulated structures in ordered (Cu–Mn)3Al alloys. Acta Metall. 1975;23:1485–500.
Marcos J, Mañosa L, Planes A, Romero R, Castro ML. Kinetics of the phase separation in Cu–Al–Mn alloys and the influence on martensitic transformations. Philos Mag. 2004;84:45–68.
Sato K, Stobbs WM. Quantification of the spinodal wave in Cu2.5Mn0.5Al by dark-field image analysis. Philos Mag A Phys Condens Matter Struct Defects Mech Prop. 1994;69:349–77.
Cuniberti A, Montecinos S, Lovey FC. Effect of γ2-phase precipitates on the martensitic transformation of a β-CuAlBe shape memory alloy. Intermetallics. 2009;17:435–40.
Velazquez D, Romero R. Spinodal decomposition and martensitic transformation in Cu–Al–Mn shape memory alloy. J Therm Anal Calorim. 2017;130:2007–13.
Wu Y, Wang J, Jiang C, Xu H. Effect of coherent nanoprecipitates on martensitic transformation in Tb-doped NiMnGa melt-spun ribbons. Intermetallics. 2018;97:42–51.
Titenko A, Demchenko L. Effect of annealing in magnetic field on ferromagnetic nanoparticle formation in Cu–Al–Mn alloy with induced martensite transformation. Nanoscale Res Lett. 2016;11:237.
Massalski T, Misutani U. Electronic structure of Hume Rothery phases. Prog Mater Sci. 1978;22:151–262.
Obradó E, Mañosa L, Planes A, Romero R, Somoza A. Quenching effects in Cu–Al–Mn shape memory alloy. Mater Sci Eng A. 1999;275:586–9.
Romero R, Somoza A, Manosa L, Planes A. Vacancies and the martensitic transition in Cu-based shape-memory alloys. A comparative study. J Phys IV Fr. 2003;112:471–4.
Lohan NM, Pricop B, Burlacu L, Bujoreanu L-G. Using DSC for the detection of diffusion-controlled phenomena in Cu-based shape memory alloys. J Therm Anal Calorim. 2016. https://doi.org/10.1007/s10973-016-5926-4.
Pishchur DP, Drebushchak VA. Recommendations on DSC calibration. J Therm Anal Calorim. 2016;124:951–8.
Sheibani S, Heshmati-Manesh S, Ataie A, Caballero A, Criado JM. Spinodal decomposition and precipitation in Cu–Cr nanocomposite. J Alloys Compd. 2014;587:670–6.
Diánez MJ, Donoso E, Sayagués MJ, Perejón A, Sánchez-Jiménez PE, Pérez-Maqueda LA, et al. The calorimetric analysis as a tool for studying the aging hardening mechanism of a Cu–10wt%Ni–5.5wt%Sn alloy. J Alloys Compd. 2016;688:288–94.
Radnóczi G, Bokányi E, Erdélyi Z, Misják F. Size dependent spinodal decomposition in Cu–Ag nanoparticles. Acta Mater. 2017;123:82–9.
Kissinger HE. Reaction Kinetics in Differential Thermal Analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose TT. Method of determining activation deteri- oration constant of electrical insulating materials. Res Rep Chiba Inst Technol (Sci Technol). 1971;16:22–31.
Svoboda R, Málek J. Is the original Kissinger equation obsolete today? J Therm Anal Calorim. 2014;117:3–7.
Stipcich M, Romero R. β-Phase thermal degradation in Zr-added Cu–Zn–Al shape memory alloy: a DSC study. J Therm Anal Calorim. 2017;129:201–7.
Zhou ZN, Yang L, Li RC, Li J, Hu QD, Li JG. Martensitic transformations and kinetics in Ni–Mn–In–Mg shape memory alloys. Intermetallics. 2018;92:49–54.
DeIasi R, Adler PN. Calorimetric studies of 7000 series aluminum alloys: I. Matrix precipitate characterization of 7075. Metall Trans A. 1977;8:1177–83.
Chen SP, Vossenberg MS, Vermolen FJ, Van De Langkruis J. Dissolution of β particles in an Al–Mg–Si alloy during DSC runs. Mater Sci Eng A. 1999;272:250–6.
Fraser RDB, Suzuki E. Resolution of overlapping absorption bands by least squares procedures. Anal Chem. 1966;38:1770–3.
Fraser RDB, Suzuki E. Resolution of overapping bands: functions for simulating band shapes. Anal Chem. 1969;41:37–9.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Santos CMA, Adorno AT, Oda NY, Sales BO, Silva LS, Silva RAG. Phase transformations and aging of the Cu72.9Al15.0Mn10.5Ag1.6 alloy. J Alloys Compd. 2016;685:587–92.
Adorno AT, Carvalho TM, Magdalena AG, Dos Santos CMA, Silva RAG. Bainitic precipitation in the Cu–9wt%Al–4wt%Mn–5wt%Ag alloy. J Alloys Compd. 2014;615:S153–5.
Liu J, Huang H, Xie J. Effects of aging treatment on the microstructure and superelasticity of columnar-grained Cu71Al18Mn11 shape memory alloy. Int J Miner Metall Mater. 2016;23:1157–66.
Recarte V, Hurtado I, Herreros J, Nó ML, San Juan J. Precipitation of the stable phases in Cu–Al–Ni shape memory alloys. Scr Mater. 1996;34:255–60.
Sepulveda A, Muñoz R, Lovey FC, Auguet C, Isalgue A, Torra V. Metastable effects on martensitic transtformation in SMA part II. The grain growth effects in Cu–Al–Be alloy. J Therm Anal. 2007;89:101–7.
Acknowledgements
This work has been carried out with the financial support of the CONICET, ANPCYT, and SECAT-UNCPBA, Argentina. The technical assistance of O. Toscano and E. Portalez in the experimental work is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Velazquez, D., Romero, R. Calorimetric study of spinodal decomposition in β-Cu–Al–Mn. J Therm Anal Calorim 143, 19–25 (2021). https://doi.org/10.1007/s10973-019-09234-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-09234-0