Abstract
The human toenail was measured in vitro in the alpha-dispersion region of the electric field and the temperature from 22 to 150 °C. The values of dielectric properties are much higher in the wet nails than in those without water of the same temperature and frequency. The peak temperature of dielectric parameters near 100 °C for wet nails is attributed to the water removal process. The dielectric spectra of the nail revealed high-frequency relaxation at 25 kHz irrespective of the water content in the tissue. Our dielectric studies of the nail plate enable deeper analysis of the matrix–keratin–water system, which can facilitate the assessment of the electrical conductivity of this tissue in the state of health and diseased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nail is an interface between living organisms and the external environment and is therefore useful as a marker of physiological changes in the structure of this tissue [1,2,3]. The influence of such external factors as relative humidity, temperature and electromagnetic radiation on the nail causes a physical–chemical release of the matrix–keratin–water composite forming this tissue. The results of our previous dielectric studies of the nail [4,5,6,7] indicate the key role of water in protecting the ordered crystalline structure of the keratin molecule before the onset of the melting process. In recent years, nails research also deals with the influence of water on the biophysical and chemical structure of this material [8,9,10,11,12].
The paper compares the thermal decomposition of the lower water content in the nail, with the decomposition of higher water content obtained in our previous studies of this material. However, our previous work on dielectric properties of nails was related to the frequency and temperature range of 100 Hz–100 kHz and 22–200 °C, respectively, which are wider than those used in this study. Earlier nail data obtained at 100 Hz were necessary for the analysis of low-frequency dispersion (LFD) and to prove that the electrode polarization does not affect the dielectric behavior of the nail below 2 kHz [6]. In this study, the lower limit of the frequency range used was set at 500 Hz, because the main purpose of this article is to show a significant difference between the size of the relaxation time of protons and active polar spaces inside the keratin above 2 kHz. Thus, the current data expand the previous analysis of the mechanism of polarization and nail conduction, taking into account the complex conductivity as an additional dielectric parameter. In addition, compared to our previous articles, the temperature range is limited to 150 °C to release loosely bound water from wet samples, which thus become dry samples for the analysis of dielectric spectra at a physiological temperature range.
Experimental
The nails of the middle fingers of the upper limbs, 3 mm wide, were obtained from the group of 15 healthy people (21–30 years). The procedure used to obtain the samples was as follows: The nails were immersed in a 0.9% NaCl solution to remove the fat, washed with distilled water, dried at room temperature (22–25 °C) with a relative humidity of about 60% and then cut into a rectangular sample about surface of 8 mm2 and a thickness of 0.3 mm.
Dielectric measurements of relative permittivity, dielectric loss and conductivity of the nail placed between the two silver paste electrodes were taken using the HIOKI 3522-50 LCR impedance analyzer in the frequency, f, range from 500 Hz to 100 kHz at temperatures, T, from 22 to 150 °C. The accuracy of the device provided by the manufacturer depends on the measuring range of the electrical impedance of the sample and the applied measuring frequency and signal level. The impedance measurement range is from 10.00 mΩ to 200.00 MΩ, and the signal level range is from 10 mV (rms) to 5 V (rms). In our experiments for 1 V (rms) nail samples in the 500 Hz–100 kHz range, the impedance values are below 200.00 MΩ, indicating that the instrument used in this test is sufficient. The method of estimating the impedance accuracy and the phase angle between the applied voltage and the current flowing through the nail on the basis of the two factors is given in the instruction table for HIOKI 3522-50 LCR HiTESTER. The accuracy of the impedance and phase angle is needed to calculate the accuracy of electrical resistance and capacitance in the parallel configuration used to determine the dielectric properties of the nail in our measurements. To obtain a high accuracy in the measurement of the electrical stability of the nail sample, the calibration procedure included in the instruction for HIOKI 3522-50 was used. Calibration of dielectric properties was obtained by measuring the properties of a Teflon sample with a known relative permittivity of 2.1, and the accuracy was estimated to be within 0.5%.
These experiments were carried out in the wet and dry states of the sample in the air atmosphere using the measuring chamber described in the previous article [13]. The wet and dry conditions concerned the same sample. Therefore, dielectric measurements include a procedure for heating a wet sample in the range of 22–150 °C and a constant temperature of 150 °C (~ 1 h) and then after rapidly cooling to room temperature, the heating procedure of the dry sample without water as a function of temperature up to 150 °C. The temperature of the nail sample was measured using a constantan-copper thermocouple with an accuracy of ΔT ~ 0.2 K. In the same measuring chamber, we also measured the water content in wet samples without electrodes. After the water removal procedure, the average water content in 5 samples (n = 5) taken from various 15 people (n = 15) participating in the study was ~ 9%. The measurements of the dielectric parameters of nail were taken for each from the 15 persons. The results for all samples are presented in the figures as average values (n = 15) and standard deviations within 5%.
Results and discussion
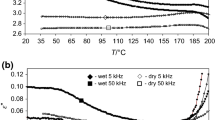
Figure 1 shows the influence of water on temperature dependence of logarithm of relative permittivity, ε′, and dielectric loss, ε″, of the nail plate at the selected frequency of 5 kHz. The release of about 9% of the water from the wet nail is manifested by ε′ and ε″ maxima near 100 °C. The curves for dry nail do not contain these peaks due to the lack of loosely bound water in this sample. Our previous dielectric studies of the human nail with about 11% water [4,5,6,7] also revealed the process of removing water, but at a lower temperature of 80 °C. Release of water in other α-keratin fibers using the DSC method [14,15,16,17] was also observed in a similar temperature range. In addition, plots for wet nail show that during the aging test for 1 h at 150 °C, the values of ε′ and ε″ are in the ranges 7.30–5.71 and 0.99–0.24, respectively. In the case of dry nail at 150 °C, the values of ε′ (5.64) and ε″ (0.22) are close to the values corresponding to the wet sample, indicating that this temperature is sufficient to release water from the nail plate. As in our previous dielectric studies on the animal horn [18] and nail [6], the results in Fig. 1 for wet samples are related to the interaction between water and polar surface groups such as OH, CO and NH keratin molecules, whereas these data for dry samples are attributed to the intermolecular interaction of the keratin inside the nail.
Figure 2 shows the frequency dependency ε′ and ε″ for wet and dry nails at 26, 36 and 45 °C referring, respectively, to the temperature before, during and above the physiological conditions. All these graphs in the full frequency range show that the dielectric parameters for wet samples are higher compared to dry samples. In other studies performed with the use of capacitive contact imaging [10] and electrical impedance spectroscopy [12], an increase in the dielectric properties of the nail was also observed along with the increase in the water content in this tissue. As a result of the presence of loosely bound water in a wet nail, two relaxation frequencies fc around 2 and 25 kHz (Fig. 2b), which are clearly visible in the case of dry nails, are masked due to joint movements between water molecules and polar active sites of keratin molecules. This overlapping effect of higher water content in the nail than in the presented paper on these two fc under physiological conditions also appeared in our earlier study [5]. In contrast, for samples without water, the relaxation processes associated with the mobility of these polar active sites of keratin molecules and surrounding protons at 2 and 25 kHz occur on the surface and inside the nail tissue, respectively. In addition, in the range of 26–45 °C, for each frequency the values of ε′ and ε″ for wet nails increase, as compared to dry samples, for which both parameters are substantially unchanged. This indicates that the process of heating wet samples breaks hydrogen bonds formed by water on the surface of keratin molecules, which leads to a higher density of relaxing free polar groups and surrounding protons in comparison to dry samples. In the spectra shown in Fig. 2, the influence of water on dielectric behavior of nails is particularly visible below 2 kHz, where there is a low-frequency dispersion (LFD) in the form ε′ ~ f−n and ε″ ~ f−k with n and k in the range of 0.03–0.06 and 0.21–0.40, respectively. Dielectric studies of other authors regarding different biopolymers [19, 20] also showed the behavior of LFD in the α-dispersion region of the electric field used for these materials. To examine the differences between wet and dry nails, dielectric data from Fig. 2 are shown in Fig. 3 as a Cole–Cole representation. For the dry nail (Fig. 3a), two relaxation processes were obtained, from the semicircle fitting, one near 2 kHz, and the other near 25 kHz. In the case of wet nails (Fig. 3b), the semicircles reflect only one frequency of relaxation near 25 kHz, and then there is a continuous monotonic increase in both ε′ and ε″ with decreasing frequency, indicating that the Cole–Cole representation is inadequate.
As shown in Fig. 4, to characterize the dielectric properties of wet and dry nails above 2 kHz, data in Fig. 2 are expressed in the form of frequency dependencies of real and imaginary parts of the complex conductivity σ* (σ* = σ′ + jσ″), where σ′ and σ″ are given by σ′ = ωεoε″ and σ″ = ωεo(ε′ − εh), respectively, εo is the permittivity of a vacuum, ω is the angular frequency (ω = 2πf), and εh is the high-frequency limit of relative permittivity at 85 kHz. These results are an extension of the previous analysis of the complex permittivity ε* (ε* = ε′ −j ε″) for the nail using the Cole–Cole function [6], as well as the data shown in Fig. 3, to demonstrate that dielectric relaxation focused around 25 kHz really exists (Fig. 4b). Conductivity plots σ′ for wet nail at temperatures of 26, 36 and 45 °C in Fig. 4a are above the respective spectra for a dry nail. This behavior is confirmed by a higher conductivity increment Δσ′ on the order of 14–18 nS cm−1 for a wet sample in the range 2–85 kHz than Δσ′ of 6 nS cm−1 for a dry sample with a small change value of this parameter with increasing temperature. Because all these plots (Fig. 4a) are expressed by σ′ ~ fp with an exponent p between 0.8 and 1, in the range of 26–45 °C, the conduction of the proton jump is the dominant mechanism of the charge carrier flow. In addition, the fact that the relaxation time τ of protons for wet and dry nails depends on temperature up to 150 °C is confirmed in Arrhenius diagrams for τ, as shown in Fig. 5. The Arrhenius equation is an empirical equation that can provide information about energy consumed in a thermally activated process. Electrical conductivity is such a process. Because the conductivity in the nail refers to the charge, the equation Arrhenius with the Boltzmann constant (k) is used as τ = το exp (ΔH/kT), where το is the pre-exponential factor and ΔH is the activation energy. Fig 5 shows the plots as the natural logarithm of the relaxation time (ln τ) relative to the inverse of the temperature (T−1). The values of τ are calculated from the equation τ = εoε∞/σ [21], where ε∞ is the high-frequency limit of α-dispersion and σ is the steady-state conductivity. In our measurements from 22 to 150 °C, we have assumed the values of σ and ε∞ at 2 kHz and 85 kHz, respectively. The activation energy ΔH of the proton conduction obtained from the slope of the plots τ to ~ 100 °C for wet nails is in the range of 0.155–0.339 eV, and above this temperature for the dry nail, the value of ΔH is 0.182 eV. Correspondingly, values of τ decrease in the range 1.30–0.16 ms for a wet sample to ~ 100 °C and 3.20–1.65 ms for a dry sample above this temperature. On the other hand, the τ values increase in the range of 2.65–3.20 ms for a dry sample up to ~ 100 °C and 0.16–0.32 ms for a wet sample above this temperature. For comparison, in the range of 22–150 °C, the maximum and minimum τ values for a dry nail are 2.5 and 10 times longer, respectively, than the corresponding values τ for a wet nail. In addition, the obtained size Δσ′ (Fig. 4a) for both nail conditions is correlated with a relaxation process of approximately 25 kHz in the dielectric σ″ spectra for wet and dry materials in Fig. 4b, as a result of simultaneous occurrence of the conduction and polarization mechanism in this tissue. This behavior is confirmed in Fig. 6 as the variation of σ″ versus σ′ for wet and dry nails with clearly visible maximum values of σ″ for fc around 25 kHz. Thus, inside the wet and dry nails, the polarization mechanism originates from the orientational relaxation of the polar intermolecular groups with a relaxation time of τc = 0.006 ms (τc = 1/2πfc, fc = 25 kHz). This value τc is shorter compared to the relaxation time τ of the proton (Fig. 5), which changes with temperature. For example, at 22 °C, τc is about 217 and 442 times shorter than the corresponding τ for wet and dry nails. These results in Fig. 6 also show that the water content in the nail influences the maximum σ″ values at any temperature while maintaining a similar relaxation frequency fc. Therefore, such a stable fc value may facilitate the assessment of physiological changes in the affected nail plate, thus indicating potentially useful dielectric spectroscopy, as well as other techniques [22,23,24,25] in medical diagnosis or therapy.
Conclusions
The presence of water in wet nail samples compared to dry samples is represented by the increase in measured dielectric parameters for each temperature and frequency of the applied electric field for these materials. During heating, partial changes in the secondary structure of keratin macromolecules are preceded by the breakage of intra- and intramolecular hydrogen bonds. In the case of wet nail samples, dielectric behavior is associated with surface polar groups such as OH, CO and NH in the side chains of amino acids as dominant interactions between the keratin–water system. As a result of the temperature increase of these samples, loosely bound water is released from the nail plate. In contrast, dry nail results are attributed to the polar regions of the intermolecular structure of this material. On the basis of dielectric measurements of the nail plate in healthy people, we can identify the proton conduction processes that affect the physiological integrity of the nail with human tissues in the clinical state.
References
Grover C, Bansal S. The nail as an investigative tool in medicine: what a dermatologist ought to know. Indian J Dermatol Venereol Leprol. 2017;83:635–43.
Bahreini M, Ashrafkhani B, Tavassoli SH. Discrimination of patients with diabetes mellitus and healthy subjects based on laser-induced breakdown spectroscopy of their fingernails. J Biomed Opt. 2013;18:1–7.
Walters KA, Abdalghafor HM, Lane ME. The human nail—barrier characterisation and permeation enhancement. Int J Pharm. 2012;435:10–21.
Jabłecka A, Olszewski J, Marzec E. Dielectric properties of keratin-water system in diabetic and healthy human fingernails. J Non Cryst Solids. 2009;355:2456–60.
Marzec E, Olszewski J. Molecular interactions in human nail plate analysed by dielectric spectroscopy. Colloids Surf B Biointerfaces. 2009;69:91–4.
Marzec E, Olszewski J. Dielectric analysis of the thermal processes of human nail. Thermochim Acta. 2010;506:73–6.
Marzec E, Wierzbicki K, Olszewski J, Samborski W, Skorupska E, Bahloul K, Krzywicka A, Krauss H. Dielectric phenomena associated with the keratin-glucose interactions of nail plate. Colloids Surf B Biointerfaces. 2013;109:143–6.
Barba C, Méndez S, Martí M, et al. Water content of hair and nails. Thermochim Acta. 2009;494:136–40.
Xiao P, Cui Y, Ciortea LI, et al. Hair and nail water holding capability measurements by using condenser—TEWL method. Int J Cosmet Sci. 2012;34:12–6.
Xiao P. Capacitive contact imaging for in vivo hair and nail water content measurements. H&PC Today. 2015;10:62–4.
Zhang H, Meng Y, Ou W, Zhang X, Liu S. Hydration of fingernail investigated by optical coherence tomography. Chin J Lasers. 2010;37:2703–8.
Benzeval I, Bowen CR, Guy RH, Delgado-Charro MB. Eeffects of iontophoresis, hydration, and permeation enhancers on human nail plate: infrared and impedance spectroscopy assessment. Pharm Res. 2013;30:1652–62.
Marzec E, Sosnowski P, Olszewski J, Krauss H, Bahloul K, Samborski W, Krawczyk-Wasielewska A. Dielectric relaxation of normothermic and hypothermic rat corneas. Bioelectrochemistry. 2015;101:132–7.
Popescu C, Augustin P, Wortmann F-J. The desorption of moisture from wool. J Therm Anal Calorim. 2015;120:113–7.
Wortmann F-J, Wortmann G, Popescu C. Kinetics of the changes imparted to the main structural components of human hair by thermal treatment. Thermochim Acta. 2018;661:78–83.
Lima CRRC, Machado LDB, Velasco MVR, Matos JR. DSC measurements applied to hair studies. J Therm Anal Calorim. 2018;132:1429–143716.
Wortmann F-J, Wortmann G, Popescu C. Linear and nonlinear relations between DSC parameters and elastic moduli for chemically and thermally treated human hair. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08252-2.
Marzec E, Kubisz L. Dielectric relaxation of air-dried horn keratin. Int J Biol Macromol. 1997;20:161–5.
Raicu V, Feldman Y. Dielectric relaxation in biological systems, physical principles, methods, and applications. Oxford: Oxford University Press; 2015.
Oliviero M, Rizvi R, Verdolotti L, Iannace S, Naguib HE, Di Maio E, Neitzert HC, Landi G. Dielectric properties of sustainable nanocomposites based on zein protein and lignin for biodegradable insulators. Adv Funct Mater. 2017;27:1–9.
Pethig R. Protein-water interactions determined by dielectric methods. Annu Rev Phys Chem. 1992;43:177–205.
Bahreini M, Hosseinimakarem Z, Tavassoli HS. A study of association between fingernail elements and osteoporosis by laser-induced breakdown spectroscopy. J Appl Phys. 2012;112:1–9.
Shi Y, Zhang J, Lu L, Shao B. Determination of triclosan and triclocarban in human nails by solid-phase extraction and ultraperformance liquid chromatography-tandem mass spectrometry. Sepu. 2013;31:1040–5.
Pearce DC, Dowling K, Gerson AR, Sim MR, Sutton SR, Newville M, et al. Arsenic microdistribution and speciation in toenail clippings of children living in a historic gold mining area. Sci Total Environ. 2010;408:2590–9.
Gherase MR, Desouza ED, Farquharson MJ, McNeill FE, Kim CY, Fleming DE, et al. X-ray fluorescence measurements of arsenic micro-distribution in human nail clippings using synchrotron radiation. Physiol Meas. 2013;34:1163–77.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Marzec, E., Olszewski, J. Influence of water and temperature on the electrical conductivity of the human nail. J Therm Anal Calorim 138, 2185–2191 (2019). https://doi.org/10.1007/s10973-019-08687-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08687-7