Abstract
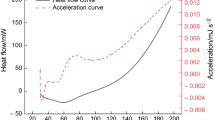
In order to further understand the effect of the water content on spontaneous combustion of coal, the thermal oxidative degradation kinetics of coals with different water contents were studied by Kissinger method, Flynn–Wall–Ozawa method, and Starink method. The results show that the water has no substantial effect on the thermal decomposition mechanism of the coal, and reaction orders are similar (n = 1) with different water contents. However, the activation energy Ea of coals has a certain relationship with the water content. When the water content of the coal is less than 6.0 mass%, with the increase of the water content, the activation energy Ea decreases. With the water content of coal exceeding 6.0 mass%, the activation energy Ea is increased with the increase of the water content. The change tendency of activation energy Ea is consistent with the mass increase of coals in the coal oxidation stage, which means that lower activation energy Ea is easier to make spontaneous combustion of coal. From this study, we hope to provide an efficient method for further understanding the mechanism of the water influence on spontaneous combustion of coal.










Similar content being viewed by others
References
Iatco C, Bostan I, Lazar C. Reconsidering economic coal resources in darfting energy strategies, the case of Romania. Environ Eng Manage J. 2013;12(10):2025–30.
Su DQ, Tang ZH, Xie JF, Bian ZG, Zhang JH, Yang DD, Zhang D, Wang JC, Liu Y, Yuan AH, Kong QH. Co, Mn-LDH nanoneedle arrays grown on Ni foam for high performance supercapacitors. Appl Surf Sci. 2019;469(5):487–94.
Chatterjee RS. Coal fire mapping from satellite thermal IR data-a case example in Jharia Coalfield, Jharkhand, India. ISPRS J Photogramm Remote Sens. 2006;60:113–28.
Pone JDN, Hein KAA, Stracher GB, Annegarn HJ, Finkleman RB, Blake DR, McCormack JK, Schroeder P. The spontaneous combustion of coal and its by-products in the Witbank and Sasolburg coalfields of South Africa. Int J Coal Geol. 2007;72(2):124–40.
Deng J, Wang K, Zhai XW. Research on the effects of high temperature environment on oxidation and spontaneous combustion characteristics. Saf Coal Min. 2014;45(3):13–5.
Wang YM, Li XQ, Wang WZ, Guo ZX. Experimental and in situ estimation on hydrogen and methane emission from spontaneous gasification in coal fire. Int J Hydrogen Energy. 2017;42(29):18728–33.
Wang JH, Zhang JS, Zhu K, Zhou L. Anatomy of explosives spontaneous combustion accidents in the Chinese underground coal mine: causes and prevention. Process Saf Prog. 2016;35(3):221–7.
Ray SK, Singh RP. Recent developments and practices to control fire in undergound coal mines. Fire Technol. 2007;43(4):285–300.
He Y. Study on microstructure variation of soaked Bitumite on low temperature oxidation process. Xian: Xian University of Science and Technology; 2016.
Feng WS. Relationship between microscopic pore structure and spontaneous combustion characteristics of coal. Shanxi: Taiyuan University of Technology; 2015.
Zhang Y, Wang J, Xue S, Wu J, Chang L, Li Z. Kinetic study on changes in methyl and methylene groups during low–temperature oxidation of coal via in situ FTIR. Int J Coal Geol. 2016;154:155–64.
Kidena K, Murakami M, Murata S, Nomura M. Low–temperature oxidation of coal suggestion of evaluation method of active methylene sites. Energy Fuels. 2003;17(4):1043–7.
Xie JJ, Yang XM, Lu XS. Research progress on the distribution and migration of sulfur and nitrogen in coal pyrolysis process. Chem Ind Eng Prog. 2004;11:1214–8.
Jones RE, Townend DTA. Oxidation of coal. J Soc Chem Ind. 1949;68(2):193–7.
Li ZH. Mechanism of free radical reactions in spontaneous combustion of coal. J China Univ Min Technol. 1996;25(3):111–4.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Tang YB, Li Y, Xue S, Wang J, Li R. Experimental investigation of long-term water immersion effect on spontaneous combustion parameters and microscopic characteristics of Bituminous. J China Coal Soc. 2017;42:2642–8.
Tang YB, Xue S. Influence of long-term water immersion on spontaneous combustion characteristics of Bulianta bituminous coal. Int J Oil Gas Coal Technol. 2017;14(4):398–411.
Yuan S, Liu JZ, Wu JH, Zhou QQ, Wang ZH, Zhou JH, Kefa C. Changes in the physicochemical characteristics and spontaneous combustion propensity of Ximeng lignite after hydrothermal dewatering. Can J Chem Eng. 2018;96(11):2387–94.
Zhang K, You CF. Effect of upgraded lignite product water content on the propensity for spontaneous ignition. Energy Fuels. 2013;27(1):20–6.
Wang H, Dlugogorski BZ, Kennedy EM. Role of inherent water in low- temperature oxidation of coal. Combust Sci Technol. 2003;175(2):253–70.
Wang H, Dlugogorski BZ, Kennedy EM. Coal oxidation at low temperatures: oxygen consumption, oxidation products, reaction mechanism and kinetic modelling. Prog Energy Combust Sci. 2003;29(6):87–113.
Zhang YT, Wang DX, Zhong XX. Study on the influence of water in low temperature oxidation of coal. Saf Coal Mines. 2007;11:1–4.
Zhang Z, Yan K. Molecular dynamics simulation of oxygen diffusion in dry and water-containing brown coal. Mol Phys. 2011;109(19):67–74.
Song S, Qin BT, Xin HH, Qin XW, Chen K. Exploring effect of water immersion on the structure and low-temperature oxidation of coal: a case study of Shendong long flame coal, China. Fuel. 2018;074(7):732–7.
Deng J, Zhao JY, Huang AC, Zhang YN, Wang CP, Shu CM. Thermal behavior and microcharacterization analysis of second-oxidized coal. J Therm Anal Calorim. 2017;1274(1):439–48.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Kuo H, Cheng F, Sahoo NG, Lu XH, Li L. Thermal kinetics of montmorillonite nanoclay/maleic anhydride-modified polypropylene nanocomposites. J Therm Anal Calorim. 2011;109:17–25.
Starink MJ. A new method for the derivation of activation energies from experiments performed at constant heating rate. Thermochim Acta. 1996;288(1/2):97–104.
Chen C, Ma X, He Y. Co-pyrolysis characteristics of microalgae chlorella vulgaris and coal through TGA. Bioresour Technol. 2012;117:264–73.
Luo R, Zhou QL. Combustion kinetic behavior of different ash contents coals co-firing with biomass and the interaction analysis. J Therm Anal Calorim. 2017;128(1):567–8.
Xu CF, Yin WB, Yao HF. Study on the optimum moisture content of coal spontaneous combustion based on activation energy index. J Saf Environ. 2018;18(4):1301–6.
Boswell PG. On the calculation of activation energies using a modified Kissinger method. J Therm Anal Calorim. 1980;18(2):353–8.
Acknowledgements
The work was financially supported by National Key R&D Program of China (2018YFC0807900), National Natural Science Foundation of China (51774014 and 51874007), Anhui Provincial Natural Science Foundation (1908085J20) and Leading Talents Project in Colleges and Universities of Anhui Province.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nie, S., Tang, M., Xing, S. et al. Investigation of water influence on coal based on thermal oxidative degradation kinetics. J Therm Anal Calorim 139, 1265–1274 (2020). https://doi.org/10.1007/s10973-019-08503-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08503-2